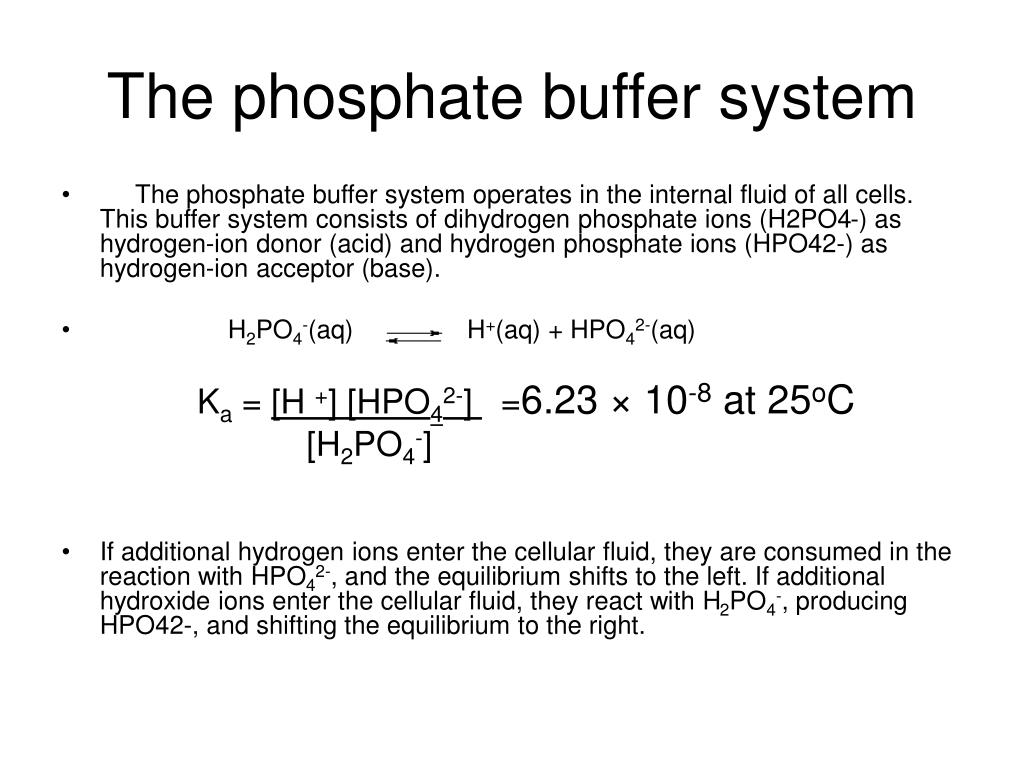
How Does The Phosphate Buffer System Work . the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system is not an important blood buffer as its concentration is too low. Proteins assist with intracellular ph. Phosphates are found in the blood in two forms: The concentration of phosphate in.
from www.slideserve.com
the phosphate buffer system is not an important blood buffer as its concentration is too low. Proteins assist with intracellular ph. Phosphates are found in the blood in two forms: the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. The concentration of phosphate in. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph.
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11
How Does The Phosphate Buffer System Work the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Proteins assist with intracellular ph. Phosphates are found in the blood in two forms: The concentration of phosphate in. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system is not an important blood buffer as its concentration is too low.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and How Does The Phosphate Buffer System Work Phosphates are found in the blood in two forms: the phosphate buffer system, while present globally, is important for the regulation of urine ph. The concentration of phosphate in. the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻). How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Experiment No 3 PowerPoint Presentation, free download ID4828328 How Does The Phosphate Buffer System Work The concentration of phosphate in. the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Azin Nowrouzi, PhD PowerPoint Presentation, free download ID How Does The Phosphate Buffer System Work the phosphate buffer system, while present globally, is important for the regulation of urine ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer system has a pk. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. The concentration of phosphate in. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Buffer Systems PowerPoint Presentation, free download ID9400078 How Does The Phosphate Buffer System Work the phosphate buffer system is not an important blood buffer as its concentration is too low. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Proteins assist with intracellular ph. Phosphates are found in the blood in two forms: the phosphate buffer works primarily through the equilibrium between. How Does The Phosphate Buffer System Work.
From www.vrogue.co
Phosphate Buffer System Equation vrogue.co How Does The Phosphate Buffer System Work Proteins assist with intracellular ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. Phosphates are found in the blood in two forms: the phosphate buffer system has a pk of 6.8, which is. How Does The Phosphate Buffer System Work.
From www.slideshare.net
Buffer system How Does The Phosphate Buffer System Work the phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; The concentration of phosphate in. the phosphate buffer system,. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT (Renal Physiology 10) AcidBase Balance 2 Buffers System How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. Phosphates are found in the. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work Proteins assist with intracellular ph. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Phosphates are found in the blood in two forms: The concentration of phosphate in. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph. How Does The Phosphate Buffer System Work.
From www.vrogue.co
Phosphate Buffer System Equation vrogue.co How Does The Phosphate Buffer System Work the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. Proteins assist with intracellular ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. The concentration of phosphate in. Phosphates are found in the blood in two forms: the phosphate buffer system has a. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: The concentration of phosphate in. the phosphate buffer system,. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint How Does The Phosphate Buffer System Work Proteins assist with intracellular ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. Phosphates are found in the blood in two forms: the phosphate buffer system, while present globally, is important for the. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11 How Does The Phosphate Buffer System Work the phosphate buffer system, while present globally, is important for the regulation of urine ph. The concentration of phosphate in. the phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms: the most relevant systems for biology are the carbonic acid/carbonate buffering system,. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the most relevant. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work The concentration of phosphate in. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Phosphates are found in the blood in two forms: the phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph. . How Does The Phosphate Buffer System Work.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit How Does The Phosphate Buffer System Work the phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the most. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Acid, Base, Electrolytes PowerPoint Presentation, free download How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Proteins assist with intracellular ph. the phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: the phosphate buffer system is. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work The concentration of phosphate in. Phosphates are found in the blood in two forms: the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download How Does The Phosphate Buffer System Work The concentration of phosphate in. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph. Phosphates are found in the blood in two forms: the phosphate buffer works primarily. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID How Does The Phosphate Buffer System Work the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Phosphates are found in the blood in two forms: The concentration of phosphate in. Proteins assist with intracellular ph. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system has. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation How Does The Phosphate Buffer System Work Phosphates are found in the blood in two forms: the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system is not an important blood buffer as. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Fluid & Electrolyte Balance PowerPoint Presentation, free How Does The Phosphate Buffer System Work Phosphates are found in the blood in two forms: The concentration of phosphate in. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system has a pk of 6.8, which is not far from. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation How Does The Phosphate Buffer System Work the phosphate buffer system, while present globally, is important for the regulation of urine ph. The concentration of phosphate in. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Phosphates are found in the blood in two forms: Proteins assist with intracellular ph. the phosphate buffer system is. How Does The Phosphate Buffer System Work.
From www.vrogue.co
Phosphate Buffer System Equation vrogue.co How Does The Phosphate Buffer System Work the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. Proteins assist with intracellular ph. The concentration of phosphate in. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells.. How Does The Phosphate Buffer System Work.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph. The concentration of phosphate in. Phosphates are found in the blood in two forms: the phosphate buffer system is. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID How Does The Phosphate Buffer System Work the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Chapter 24 Water, Electrolyte and AcidBase Balance PowerPoint How Does The Phosphate Buffer System Work The concentration of phosphate in. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system is not an important blood buffer as its concentration is too low. Proteins assist with intracellular ph. the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Phosphates are found in the blood in two forms: the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer works primarily through the. How Does The Phosphate Buffer System Work.
From www.slideshare.net
Ch 26 How Does The Phosphate Buffer System Work the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system has a pk of 6.8,. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Proteins assist with intracellular ph. The concentration of phosphate in. the phosphate buffer system is not an important blood buffer as its concentration is too low. Phosphates are found in the blood in two forms:. How Does The Phosphate Buffer System Work.
From ar.inspiredpencil.com
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the phosphate buffer works primarily through the equilibrium between dihydrogen phosphate (h₂po₄⁻) and hydrogen. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system has a pk of 6.8,. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the phosphate buffer system is not an important blood buffer as its concentration is too low. the phosphate buffer system, while present globally, is important for the regulation of urine ph. Proteins assist with intracellular ph. the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the. How Does The Phosphate Buffer System Work.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download How Does The Phosphate Buffer System Work the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Phosphates are found in the blood in two forms: the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system is not an important blood buffer as its concentration is too. How Does The Phosphate Buffer System Work.
From www.youtube.com
Phosphate buffer system (idea). YouTube How Does The Phosphate Buffer System Work the phosphate buffer system has a pk of 6.8, which is not far from the normal ph of 7.4 in the body fluids; Proteins assist with intracellular ph. the phosphate buffer system, while present globally, is important for the regulation of urine ph. the phosphate buffer system is not an important blood buffer as its concentration is. How Does The Phosphate Buffer System Work.
From animalia-life.club
Phosphate Buffer System Equation How Does The Phosphate Buffer System Work the most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. the phosphate buffer system is not an important blood buffer as its concentration is too low. Proteins assist with intracellular ph. Phosphates are found in the blood in two forms: the phosphate buffer system has a pk of 6.8,. How Does The Phosphate Buffer System Work.