Tin-124 Protons Neutrons Electrons . These all consist of an atomic nucleus with 50. 124 (= number of nucleons). Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. electron configuration of tin. Tin isotopes are used in a variety of applications. 124 sn or 12450 sn mass number a: the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has the most stable isotopes (10) of all elements. Electron configuration can be done in two ways. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic.
from www.youtube.com
Tin has the most stable isotopes (10) of all elements. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Tin isotopes are used in a variety of applications. These all consist of an atomic nucleus with 50. 124 (= number of nucleons). all atomic nuclei of the chemical element tin are summarized under tin isotopes; the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. electron configuration of tin. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10.
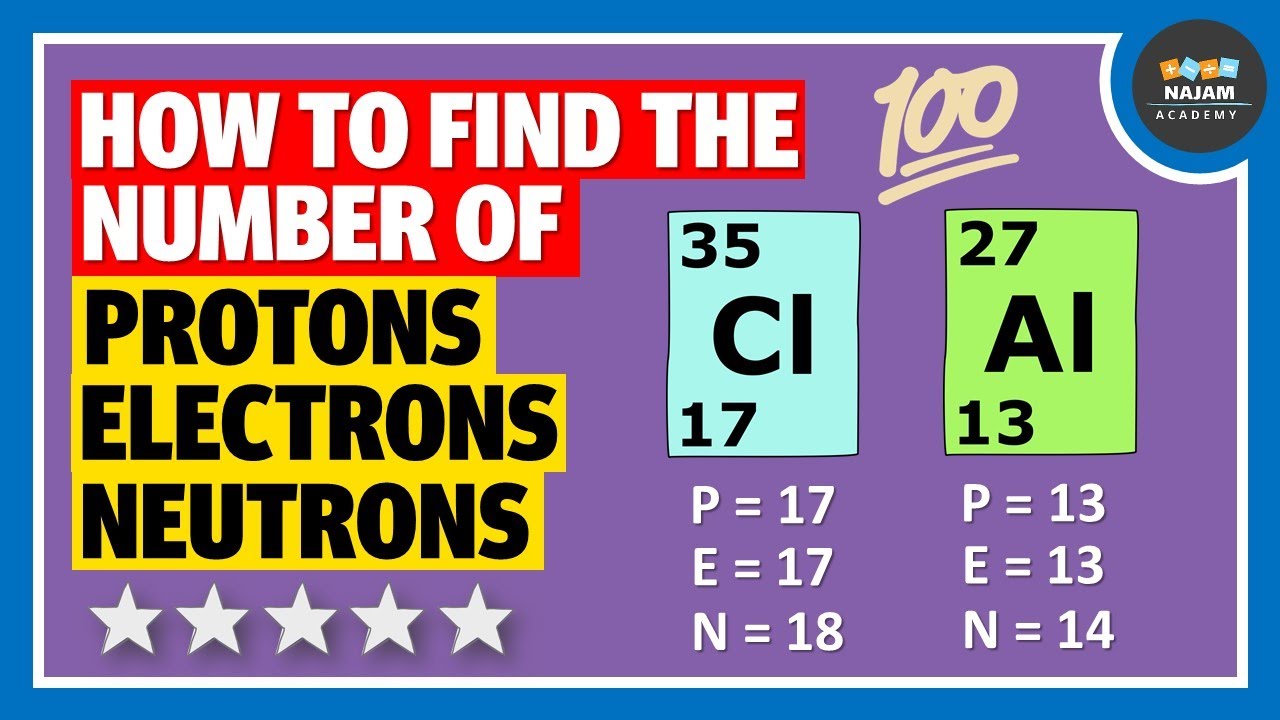
How to find the number of Protons, Neutrons and Electrons? Chemistry
Tin-124 Protons Neutrons Electrons electron configuration of tin. These all consist of an atomic nucleus with 50. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. all atomic nuclei of the chemical element tin are summarized under tin isotopes; electron configuration of tin. Tin has the most stable isotopes (10) of all elements. 124 sn or 12450 sn mass number a: 124 (= number of nucleons). tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) Tin isotopes are used in a variety of applications. Electron configuration can be done in two ways.
From www.nuclear-power.com
Tin Atomic Number Atomic Mass Density of Tin Tin-124 Protons Neutrons Electrons the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. 124 (= number of nucleons). electron configuration of tin. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Electron configuration can be done in two. Tin-124 Protons Neutrons Electrons.
From printablefulltacet.z19.web.core.windows.net
Proton Neutron Electron Charge Worksheet Tin-124 Protons Neutrons Electrons Tin isotopes are used in a variety of applications. These all consist of an atomic nucleus with 50. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. all atomic nuclei of the chemical element tin are summarized under tin isotopes; tin is a chemical element with. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tin (Sn, Sn2+, Sn4+) Tin-124 Protons Neutrons Electrons Electron configuration can be done in two ways. electron configuration of tin. 124 sn or 12450 sn mass number a: Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. These all consist of an atomic nucleus with 50. Tin has. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin-124 Protons Neutrons Electrons Tin isotopes are used in a variety of applications. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 124 sn or 12450 sn mass number a: Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) Tin has a. Tin-124 Protons Neutrons Electrons.
From www.chegg.com
Solved Consider the tin124 isotope and complete the numbers Tin-124 Protons Neutrons Electrons tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Tin-124 Protons Neutrons Electrons.
From www.shutterstock.com
Bohr Model Tin Atom Electron Structure Stock Vector (Royalty Free Tin-124 Protons Neutrons Electrons Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Electron configuration can be done in two ways. Tin has the most stable isotopes (10) of all elements. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2,. Tin-124 Protons Neutrons Electrons.
From www.chegg.com
Solved Consider the tin124 isotope and complete the numbers Tin-124 Protons Neutrons Electrons all atomic nuclei of the chemical element tin are summarized under tin isotopes; 124 sn or 12450 sn mass number a: Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. tin is a chemical element with atomic number 50. Tin-124 Protons Neutrons Electrons.
From utedzz.blogspot.com
Periodic Table Of Elements List With Protons Neutrons And Electrons Tin-124 Protons Neutrons Electrons the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s. Tin-124 Protons Neutrons Electrons.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin-124 Protons Neutrons Electrons electron configuration of tin. all atomic nuclei of the chemical element tin are summarized under tin isotopes; the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. 124 sn or 12450 sn mass number a: Electron configuration can be done in two ways. Tin isotopes are used. Tin-124 Protons Neutrons Electrons.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry Tin-124 Protons Neutrons Electrons all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has the most stable isotopes (10) of all elements. Tin isotopes are used in a variety of applications. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Tin has a ground state. Tin-124 Protons Neutrons Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Sn (Tin Tin-124 Protons Neutrons Electrons all atomic nuclei of the chemical element tin are summarized under tin isotopes; These all consist of an atomic nucleus with 50. Electron configuration can be done in two ways. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. 124. Tin-124 Protons Neutrons Electrons.
From printablelibcoulter.z21.web.core.windows.net
Electrons Protons And Neutrons Charges Tin-124 Protons Neutrons Electrons electron configuration of tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. 124 (= number of nucleons). the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. . Tin-124 Protons Neutrons Electrons.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Tin-124 Protons Neutrons Electrons Electron configuration can be done in two ways. electron configuration of tin. These all consist of an atomic nucleus with 50. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. 124 (= number of nucleons). 124 sn or 12450 sn mass number a: Tin has a ground. Tin-124 Protons Neutrons Electrons.
From circuitwiringblunts77.z22.web.core.windows.net
Diagram Of Protons Neutrons And Electrons Tin-124 Protons Neutrons Electrons These all consist of an atomic nucleus with 50. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. Tin isotopes are used in a variety of applications. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through. Tin-124 Protons Neutrons Electrons.
From www.slidemake.com
Subatomic Particles Presentation Tin-124 Protons Neutrons Electrons Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) 124 (= number of nucleons). These all consist of an atomic nucleus with 50. all atomic nuclei of the chemical element tin are summarized under tin isotopes; electron configuration of tin. Electron configuration can be done in two ways. Tin. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin-124 Protons Neutrons Electrons Tin has the most stable isotopes (10) of all elements. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 124 sn or 12450 sn mass number a: Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Tin-124 Protons Neutrons Electrons.
From hxecktzfj.blob.core.windows.net
Electron Configuration Chart Of Tin at Allison Napolitano blog Tin-124 Protons Neutrons Electrons Electron configuration can be done in two ways. These all consist of an atomic nucleus with 50. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. 124 (= number of nucleons). tin is a chemical element with atomic number 50. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin-124 Protons Neutrons Electrons the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) electron configuration of tin. 124 (= number of nucleons). These all consist of an atomic nucleus with 50. tin. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tin (Sn, Sn2+, Sn4+) Tin-124 Protons Neutrons Electrons 124 (= number of nucleons). 124 sn or 12450 sn mass number a: Tin isotopes are used in a variety of applications. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr. Tin-124 Protons Neutrons Electrons.
From www.slideserve.com
PPT How to find the number of protons, neutrons, & electrons for an Tin-124 Protons Neutrons Electrons These all consist of an atomic nucleus with 50. all atomic nuclei of the chemical element tin are summarized under tin isotopes; Electron configuration can be done in two ways. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. electron configuration of tin. 124 sn or. Tin-124 Protons Neutrons Electrons.
From owenholt.z21.web.core.windows.net
Element Proton Neutron Electron Chart Tin-124 Protons Neutrons Electrons 124 (= number of nucleons). Tin has the most stable isotopes (10) of all elements. These all consist of an atomic nucleus with 50. Tin isotopes are used in a variety of applications. all atomic nuclei of the chemical element tin are summarized under tin isotopes; tin is a chemical element with atomic number 50 which means there. Tin-124 Protons Neutrons Electrons.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin-124 Protons Neutrons Electrons electron configuration of tin. These all consist of an atomic nucleus with 50. 124 (= number of nucleons). Tin isotopes are used in a variety of applications. Tin has the most stable isotopes (10) of all elements. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Tin-124 Protons Neutrons Electrons.
From www.sliderbase.com
The Periodic Table the Crust Tin-124 Protons Neutrons Electrons tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. electron configuration of tin. 124 (= number of nucleons). Tin has the most stable isotopes (10) of all elements. Tin isotopes are used in a variety of applications. 124 sn or 12450 sn mass number a: . Tin-124 Protons Neutrons Electrons.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin-124 Protons Neutrons Electrons tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. These all consist of an atomic nucleus with 50. all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s. Tin-124 Protons Neutrons Electrons.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin-124 Protons Neutrons Electrons Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. 124 (= number of nucleons). electron configuration of tin. Tin has the most stable isotopes (10) of all elements. These all consist of an atomic nucleus with 50. Electron configuration can. Tin-124 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Electron Proton Neutron Tin-124 Protons Neutrons Electrons Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 124 (= number of nucleons). the electron configuration of. Tin-124 Protons Neutrons Electrons.
From quizzschoolfischer.z13.web.core.windows.net
Protons Neutrons And Electrons Worksheets Tin-124 Protons Neutrons Electrons Tin isotopes are used in a variety of applications. 124 sn or 12450 sn mass number a: Electron configuration can be done in two ways. Tin has the most stable isotopes (10) of all elements. the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. all atomic nuclei. Tin-124 Protons Neutrons Electrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Tin-124 Protons Neutrons Electrons These all consist of an atomic nucleus with 50. 124 (= number of nucleons). electron configuration of tin. Tin has the most stable isotopes (10) of all elements. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. the electron. Tin-124 Protons Neutrons Electrons.
From ar.inspiredpencil.com
Tin Atomic Structure Tin-124 Protons Neutrons Electrons Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) Tin has the most stable isotopes (10) of all elements. 124 (= number of nucleons). Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d. Tin-124 Protons Neutrons Electrons.
From schematicdatavenin77.z5.web.core.windows.net
Diagram Of Protons Neutrons And Electrons Tin-124 Protons Neutrons Electrons the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Tin isotopes are used in a variety of applications. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Tin-124 Protons Neutrons Electrons.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin-124 Protons Neutrons Electrons 124 (= number of nucleons). Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. 124 sn or 12450 sn mass number a: These all consist of an atomic nucleus. Tin-124 Protons Neutrons Electrons.
From www.numerade.com
How many electrons, protons, and neutrons are there in each of the Tin-124 Protons Neutrons Electrons Tin isotopes are used in a variety of applications. 124 sn or 12450 sn mass number a: the electron configuration of tin is [kr] 4d 10 5s 2 5p 2, if the electron arrangement is through orbitals. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Tin-124 Protons Neutrons Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Compare Sodium vs Tin Periodic Table Element Comparison Compare Tin-124 Protons Neutrons Electrons 124 (= number of nucleons). These all consist of an atomic nucleus with 50. Electron configuration can be done in two ways. Tin has the most stable isotopes (10) of all elements. 124 sn or 12450 sn mass number a: Tin isotopes are used in a variety of applications. electron configuration of tin. Tin has a ground state electron. Tin-124 Protons Neutrons Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin-124 Protons Neutrons Electrons Electron configuration can be done in two ways. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) tin atom electron configuration (bohr model) These all consist of an atomic nucleus with 50. 124 sn or 12450 sn mass number a: all atomic nuclei of the chemical element tin are summarized under tin isotopes; Tin has a. Tin-124 Protons Neutrons Electrons.
From www.slideserve.com
PPT Tremendous Tin PowerPoint Presentation, free download ID2598669 Tin-124 Protons Neutrons Electrons These all consist of an atomic nucleus with 50. Electron configuration can be done in two ways. tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic. all atomic nuclei of the chemical element tin are summarized under tin isotopes; 124 (= number of nucleons). electron. Tin-124 Protons Neutrons Electrons.