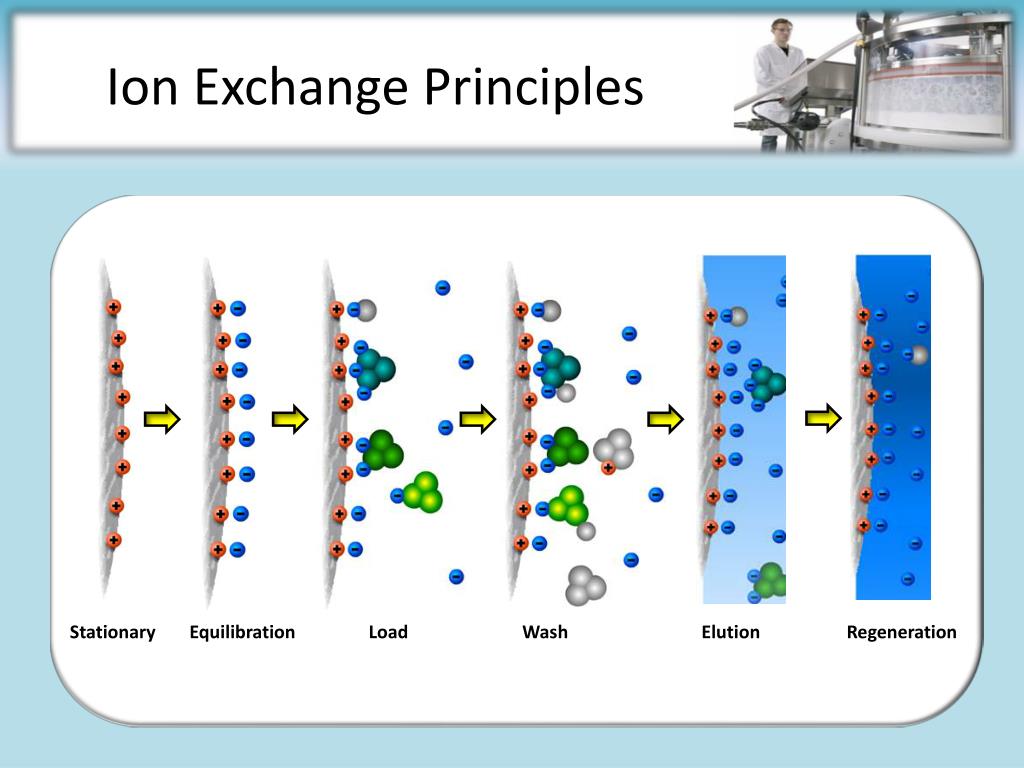
Ion Exchange Explained . It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules.
from www.slideserve.com
The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. It is often used for inorganic anions.
PPT IEX Chromatography PowerPoint Presentation, free download ID
Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. Protein charge depends on the number. It is often used for inorganic anions.
From design.udlvirtual.edu.pe
Ion Exchange Process Design Design Talk Ion Exchange Explained Protein charge depends on the number. This technique is used to analyze ionic substances. It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From mytapscore.com
How Does Ion Exchange Work? SimpleLab Tap Score Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Protein charge depends on the number. This technique is used to analyze ionic substances. Ion Exchange Explained.
From pnghut.com
Ion Exchange Process Flow Diagram Ionexchange Resin Separation Ion Exchange Explained Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion Exchange Explained.
From www.youtube.com
Ion exchange chromatography YouTube Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Protein charge depends on the number. This technique is used to analyze ionic substances. Ion Exchange Explained.
From www.slideserve.com
PPT Ion exchange chromatography PowerPoint Presentation, free Ion Exchange Explained Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion Exchange Explained.
From www.youtube.com
ION EXCHANGE PROCESS YouTube Ion Exchange Explained This technique is used to analyze ionic substances. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion Exchange Explained.
From www.youtube.com
Introduction to Ionexchange chromatography YouTube Ion Exchange Explained This technique is used to analyze ionic substances. Protein charge depends on the number. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From waterfilterguru.com
What is Ion Exchange? Water Filter Guru Ion Exchange Explained This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. Ion Exchange Explained.
From www.youtube.com
Ion chromatography (Ion pair and Ion Exchange chromatography) basic Ion Exchange Explained Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. It is often used for inorganic anions. Ion Exchange Explained.
From www.youtube.com
Ion Exchange Chromatography Theory and Principle YouTube Ion Exchange Explained Protein charge depends on the number. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From www.shimadzu.com
Ion Exchange Chromatography SHIMADZU (Shimadzu Corporation) Ion Exchange Explained This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From exyeaqpsg.blob.core.windows.net
Ion Exchange For Dummies at Ashley Glynn blog Ion Exchange Explained This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is often used for inorganic anions. Ion Exchange Explained.
From treatment-faq.com
How Does Ion Exchange In Water Treatment Work Ion Exchange Explained This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. Ion Exchange Explained.
From www.slideserve.com
PPT FUNDAMENTAL OF ION EXCHANGE PowerPoint Presentation, free Ion Exchange Explained Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion Exchange Explained.
From hydroservice.com
Ion Exchange Explained Hydro Services Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. It is often used for inorganic anions. Protein charge depends on the number. Ion Exchange Explained.
From www.slideshare.net
Ion Exchange Chromatography, ppt Ion Exchange Explained This technique is used to analyze ionic substances. Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is often used for inorganic anions. Ion Exchange Explained.
From www.slideshare.net
10 ion exchange process Ion Exchange Explained Protein charge depends on the number. It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From www.slideserve.com
PPT IEX Chromatography PowerPoint Presentation, free download ID Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Protein charge depends on the number. Ion Exchange Explained.
From design.udlvirtual.edu.pe
Ion Exchange Chromatography Definition And Types Design Talk Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. It is often used for inorganic anions. Ion Exchange Explained.
From design.udlvirtual.edu.pe
Cation Exchange Chromatography Explained Design Talk Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. Protein charge depends on the number. It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From rephile.com
Ion Exchange Method RephiLe Bioscience Ion Exchange Explained Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. It is often used for inorganic anions. Ion Exchange Explained.
From www.youtube.com
PWNT'S developed SIX® (Suspended Ion eXchange) water treatment process Ion Exchange Explained It is often used for inorganic anions. This technique is used to analyze ionic substances. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From microbenotes.com
Ion Exchange Chromatography Principle, Parts, Steps, Uses Ion Exchange Explained It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From www.youtube.com
Principles of Ion exchange chromatography YouTube Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Protein charge depends on the number. It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From www.water-rightgroup.com
How Water Softeners Work and Why You Need One WaterRight Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. It is often used for inorganic anions. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From pt.slideshare.net
Ion exchange chromatography Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. It is often used for inorganic anions. Protein charge depends on the number. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From www.slidemake.com
Ion Exchange Chromatography Presentation Ion Exchange Explained It is often used for inorganic anions. Protein charge depends on the number. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From www.intechopen.com
Figure 3. Ion Exchange Explained Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. Ion Exchange Explained.
From www.youtube.com
The Principle Of Ion Exchange Chromatography, A Full Explanation YouTube Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is often used for inorganic anions. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. This technique is used to analyze ionic substances. Protein charge depends on the number. Ion Exchange Explained.
From www.researchgate.net
Schematic illustration of ionexchange chromatography. The Ion Exchange Explained It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From exyeaqpsg.blob.core.windows.net
Ion Exchange For Dummies at Ashley Glynn blog Ion Exchange Explained Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. It is often used for inorganic anions. This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Protein charge depends on the number. Ion Exchange Explained.
From medium.com
Applications of ion exchange chromatography Study Chemistry Ion Exchange Explained Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Ion Exchange Explained.
From www.seplite.com
SEPLIFE®, All You Need to Know about Ion Exchange Chromatography Sunresin Ion Exchange Explained The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. This technique is used to analyze ionic substances. Protein charge depends on the number. Ion Exchange Explained.
From www.fuelcellstore.com
of Ion Exchange Materials Ion Exchange Explained It is often used for inorganic anions. This technique is used to analyze ionic substances. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. Ion Exchange Explained.
From mytapscore.com
How Does Ion Exchange Work? SimpleLab Tap Score Ion Exchange Explained This technique is used to analyze ionic substances. The soluble, ionised substances are present in water as ions, which are electrically charged atoms or molecules. It is often used for inorganic anions. Ion exchange chromatography is the reversible adsorption of charged molecules to immobilized ion groups on a matrix of an opposite charge. Protein charge depends on the number. Ion Exchange Explained.