Emission Spectra Definition Physics . In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. When an electron jumps from a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. The actual wavelengths of the lines are predictably.
from chemistrypuns-periodically.weebly.com
Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. The actual wavelengths of the lines are predictably. When an electron jumps from a. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum.
Chemistry Electron Emission Spectrum
Emission Spectra Definition Physics Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The actual wavelengths of the lines are predictably. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. When an electron jumps from a.
From wisc.pb.unizin.org
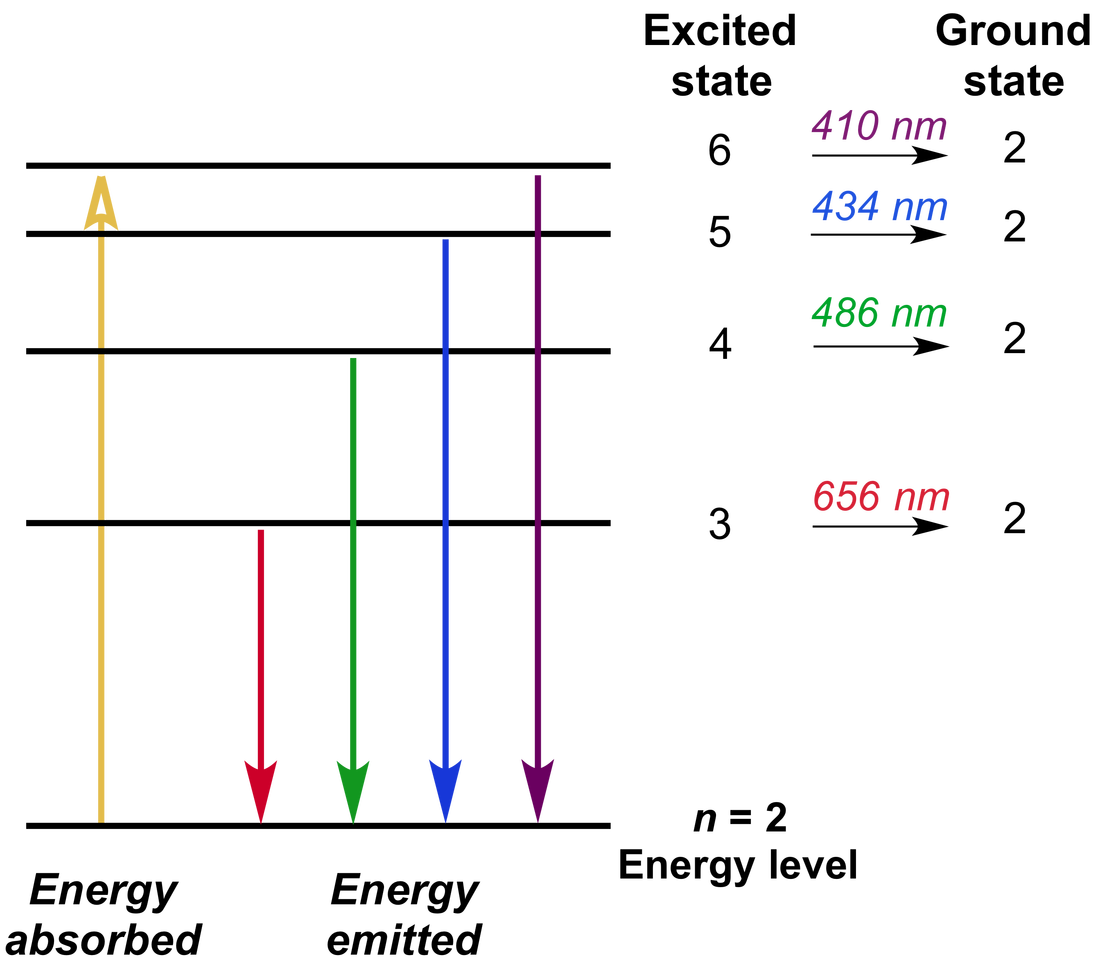
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Definition Physics The actual wavelengths of the lines are predictably. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. When an electron jumps from a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This spectrum of radiation emitted by electrons in the excited. Emission Spectra Definition Physics.
From www.vrogue.co
Define Emission Spectron And Absorption Spectrum vrogue.co Emission Spectra Definition Physics The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When an electron jumps from a. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light.. Emission Spectra Definition Physics.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This spectrum of radiation emitted by electrons in the excited. Emission Spectra Definition Physics.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Definition Physics Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The actual wavelengths of the lines are predictably.. Emission Spectra Definition Physics.
From www.savemyexams.com
Emission & Absorption Spectra in Stars AQA A Level Physics Revision Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When an electron jumps from a. The actual wavelengths of the lines are predictably. Emission spectra are patterns of light emitted by atoms or. Emission Spectra Definition Physics.
From materiallibrarycagle.z13.web.core.windows.net
What Does An Emission Spectra Show Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When an electron jumps from a. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to. Emission Spectra Definition Physics.
From byjus.com
What do you mean by spectrum? Give the complete Emission Spectra Definition Physics Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When an electron jumps from a. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in. Emission Spectra Definition Physics.
From www.slideserve.com
PPT Atomic Emission Spectrum PowerPoint Presentation, free download Emission Spectra Definition Physics Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The actual wavelengths of the lines are predictably. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Emission spectra are the spectrum of light released. Emission Spectra Definition Physics.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The actual wavelengths of the lines are predictably. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. Atomic emission spectra refer to the distinct lines. Emission Spectra Definition Physics.
From whitsonmysecutage.blogspot.com
Continuous Spectrum Vs Emission Spectrum Vs Absorption Spectrum Emission Spectra Definition Physics Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state. Emission Spectra Definition Physics.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a. Emission Spectra Definition Physics.
From testbook.com
Emission Spectrum Definition, Types, Atomic Spectra of Hydrogen Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic spectra refer. Emission Spectra Definition Physics.
From byjus.com
Atomic Spectra (Emission Spectrum & Absorption Spectra) Detailed Emission Spectra Definition Physics When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are patterns of light emitted by atoms or molecules when they. Emission Spectra Definition Physics.
From www.slideserve.com
PPT EMISSION AND ABSORPTION SPECTRA PowerPoint Presentation, free Emission Spectra Definition Physics The actual wavelengths of the lines are predictably. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. When an electron jumps from a.. Emission Spectra Definition Physics.
From www.youtube.com
A Level Physics Absorption and emission spectra explained YouTube Emission Spectra Definition Physics Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as. Emission Spectra Definition Physics.
From www.researchgate.net
a emission spectra; b normalized emission spectra; c UV spectrum (left Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are the spectrum of light released from excited atoms or molecules as. Emission Spectra Definition Physics.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum.. Emission Spectra Definition Physics.
From www.britannica.com
Emission physics Britannica Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission. Emission Spectra Definition Physics.
From www.congress-intercultural.eu
Types Of Spectra Continuous, Emission, And Absorption, 53 OFF Emission Spectra Definition Physics The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. When an electron jumps from a. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated. Emission Spectra Definition Physics.
From www.slideserve.com
PPT Chapter 4 Electron Configurations and Quantum Chemistry Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. The actual wavelengths of the lines are predictably. Atomic spectra refer to the unique patterns of light emitted or absorbed by. Emission Spectra Definition Physics.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Definition Physics The actual wavelengths of the lines are predictably. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. Atomic spectra refer to the unique patterns of light emitted. Emission Spectra Definition Physics.
From www.slideserve.com
PPT Quantum physics (quantum theory, quantum mechanics) PowerPoint Emission Spectra Definition Physics When an electron jumps from a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. Emission. Emission Spectra Definition Physics.
From waves.neocities.org
The Spectrum Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Emission spectra are the spectrum of light released from excited. Emission Spectra Definition Physics.
From animalia-life.club
Bohr Model Of Hydrogen Emission Spectrum Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When an electron jumps from a. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. Atomic emission spectra refer to the distinct lines or bands of. Emission Spectra Definition Physics.
From www.pinterest.co.uk
Image of absorption, emission, and continuous spectra. Absorption Emission Spectra Definition Physics Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their. Emission Spectra Definition Physics.
From www.youtube.com
What is the Difference Between Absorption and Emission Spectra Atomic Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. The spectroscope separates the light of varying wavelengths and we observe a line spectrum.. Emission Spectra Definition Physics.
From www.sliderbase.com
Emission spectra Presentation Physics Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This spectrum of radiation emitted by electrons in the excited atoms or molecules is. Emission Spectra Definition Physics.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When an electron jumps from a. Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of. Emission Spectra Definition Physics.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to. Emission Spectra Definition Physics.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Emission Spectra Definition Physics When an electron jumps from a. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom. Emission Spectra Definition Physics.
From cocoqas.weebly.com
Difference between emission and absorption spectra cocoQas Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. The spectroscope separates the light of varying wavelengths and we observe a line spectrum.. Emission Spectra Definition Physics.
From chem.libretexts.org
6.4 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. Atomic emission spectra refer to the distinct lines or bands of color. Emission Spectra Definition Physics.
From www.slideserve.com
PPT XRAY EMISSION SPECTRUM PowerPoint Presentation, free download Emission Spectra Definition Physics This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher. Emission Spectra Definition Physics.
From www.thoughtco.com
Balmer Series Definition in Science Emission Spectra Definition Physics Atomic emission spectra refer to the distinct lines or bands of color produced when atoms release energy in the form of light. Emission spectra are patterns of light emitted by atoms or molecules when they transition from a higher energy state to a lower energy state. When an electron jumps from a. The spectroscope separates the light of varying wavelengths. Emission Spectra Definition Physics.
From study.com
Continuous Spectrum Definition, Types & Examples Lesson Emission Spectra Definition Physics In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Emission spectra are the spectrum of light released from excited atoms or molecules as they return to lower energy states. When an electron jumps from a. The spectroscope separates the light of varying wavelengths and we. Emission Spectra Definition Physics.