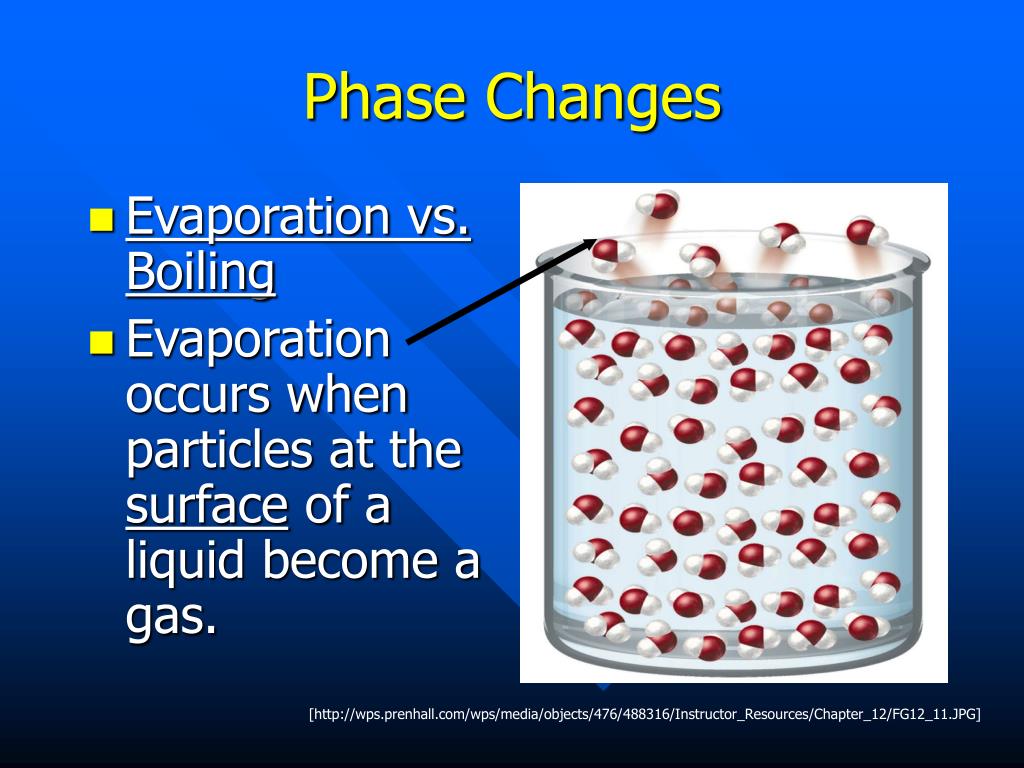
What Is A Evaporation Phase Change . In melting (or “ fusion ”), a solid turns into. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Solve calorimetry problems involving phase changes. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Phase transitions play an important theoretical and practical role in the study of heat flow. Condensation is the change of state.
from www.slideserve.com
In melting (or “ fusion ”), a solid turns into. Phase transitions play an important theoretical and practical role in the study of heat flow. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Condensation is the change of state. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Solve calorimetry problems involving phase changes. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change.
PPT Phase Changes PowerPoint Presentation, free download ID5049190
What Is A Evaporation Phase Change Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Phase transitions play an important theoretical and practical role in the study of heat flow. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into. Condensation is the change of state.
From www.expii.com
Vaporization — Definition & Overview Expii What Is A Evaporation Phase Change For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Condensation is the change of state. Phase transitions play an important theoretical and practical role in the study of heat flow. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Solve calorimetry. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5359094 What Is A Evaporation Phase Change Define melting, freezing, vaporization, condensation, sublimation, and deposition. Phase transitions play an important theoretical and practical role in the study of heat flow. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the change of state. For starters, evaporation is the process by which liquid water molecules break the bonds. What Is A Evaporation Phase Change.
From www.worksheetsplanet.com
Phase Changes of Matter What Is A Evaporation Phase Change For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Define melting, freezing, vaporization, condensation, sublimation, and deposition. In melting (or “ fusion ”), a solid turns into. Phase transitions play an important theoretical and practical role in the study of. What Is A Evaporation Phase Change.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) What Is A Evaporation Phase Change Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Condensation is the change of state. Evaporation is the conversion of a liquid to its vapor below the. What Is A Evaporation Phase Change.
From quizlet.com
Evaporation Diagram Quizlet What Is A Evaporation Phase Change Phase transitions play an important theoretical and practical role in the study of heat flow. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous. What Is A Evaporation Phase Change.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids What Is A Evaporation Phase Change In melting (or “ fusion ”), a solid turns into. Condensation is the change of state. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Phase transitions play an important theoretical and practical role in the study of heat flow.. What Is A Evaporation Phase Change.
From www.researchgate.net
Phase change diagram temperature as a function of heat added. T m and What Is A Evaporation Phase Change In melting (or “ fusion ”), a solid turns into. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Evaporation is the. What Is A Evaporation Phase Change.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii What Is A Evaporation Phase Change Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the change of state. Phase transitions play an important theoretical and practical role in the study of heat flow. Define melting, freezing, vaporization, condensation, sublimation, and deposition. In melting (or “ fusion ”), a solid turns into. Instead, the additional thermal. What Is A Evaporation Phase Change.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation What Is A Evaporation Phase Change Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Solve calorimetry problems involving phase changes.. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free What Is A Evaporation Phase Change For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. In melting (or “ fusion ”), a solid turns into. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation, process by which. What Is A Evaporation Phase Change.
From www.researchgate.net
Phase Changes in Water (A) Interfacial solar evaporation. Reproduced What Is A Evaporation Phase Change Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation, process by which. What Is A Evaporation Phase Change.
From education-portal.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation What Is A Evaporation Phase Change Phase transitions play an important theoretical and practical role in the study of heat flow. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous. What Is A Evaporation Phase Change.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. Condensation. What Is A Evaporation Phase Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Is A Evaporation Phase Change Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. Condensation is the change of state. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Solve calorimetry problems involving phase changes. For starters, evaporation is the. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. In melting (or “ fusion ”), a solid turns into. Phase transitions play an important theoretical and practical role in the study of heat flow. Solve calorimetry problems involving phase changes. Define melting, freezing, vaporization, condensation, sublimation, and deposition.. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID6811853 What Is A Evaporation Phase Change In melting (or “ fusion ”), a solid turns into. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Solve calorimetry problems involving phase changes. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Chapter 17 CHANGE OF PHASE PowerPoint Presentation, free What Is A Evaporation Phase Change Phase transitions play an important theoretical and practical role in the study of heat flow. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Define melting, freezing, vaporization, condensation, sublimation, and. What Is A Evaporation Phase Change.
From www.britannica.com
Phase Definition & Facts Britannica What Is A Evaporation Phase Change Solve calorimetry problems involving phase changes. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Instead, the additional thermal. What Is A Evaporation Phase Change.
From tutors.com
Evaporation Definition & Examples (Video) What Is A Evaporation Phase Change For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Solve calorimetry problems involving phase changes. Condensation is the change of state. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation, process by which an element or compound transitions from its. What Is A Evaporation Phase Change.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Condensation is the change of state. Phase transitions play an important theoretical and practical role in the study of heat flow. Define. What Is A Evaporation Phase Change.
From www.pinterest.com
Changes of states. Part 2 of 6. Evaporation water boiling. Phase What Is A Evaporation Phase Change Phase transitions play an important theoretical and practical role in the study of heat flow. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Solve calorimetry problems involving phase changes. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. For starters, evaporation is the process by which liquid water molecules break the. What Is A Evaporation Phase Change.
From www.slideshare.net
Phase changes What Is A Evaporation Phase Change In melting (or “ fusion ”), a solid turns into. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at.. What Is A Evaporation Phase Change.
From apollo.nvu.vsc.edu
Latent Heat of evaporation, fusion, and freezing What Is A Evaporation Phase Change Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Instead, the additional thermal energy acts to loosen bonds between. What Is A Evaporation Phase Change.
From guides.co
Phase Change Requires Heat FD2021 Fundamentals of Fire and What Is A Evaporation Phase Change Phase transitions play an important theoretical and practical role in the study of heat flow. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the. What Is A Evaporation Phase Change.
From www.worldatlas.com
The Water Cycle WorldAtlas What Is A Evaporation Phase Change Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Phase transitions play an important theoretical and practical role in the study of heat flow. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. In melting (or “ fusion ”), a. What Is A Evaporation Phase Change.
From www.dreamstime.com
Water States of Matter Phase. Change of State for Water Diagram Stock What Is A Evaporation Phase Change Define melting, freezing, vaporization, condensation, sublimation, and deposition. Condensation is the change of state. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor,. What Is A Evaporation Phase Change.
From www.geeksforgeeks.org
What is Vaporization? What Is A Evaporation Phase Change For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. Solve calorimetry problems involving phase changes. Condensation is the change of state. In melting (or “ fusion ”), a solid turns into. Evaporation, process by which an element or compound transitions. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phases, Phase Changes, Chemical and Physical Changes PowerPoint What Is A Evaporation Phase Change Define melting, freezing, vaporization, condensation, sublimation, and deposition. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Solve calorimetry problems involving phase changes. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor,. What Is A Evaporation Phase Change.
From nursehub.com
Phase Changes NurseHub What Is A Evaporation Phase Change Solve calorimetry problems involving phase changes. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Condensation is the change of state. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor, and as i mentioned. In melting (or “ fusion ”), a solid turns into. Evaporation. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation Phase Change Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. In melting (or “ fusion ”), a solid turns into. Phase transitions play an important theoretical and practical role in the study of heat flow. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change.. What Is A Evaporation Phase Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Is A Evaporation Phase Change Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. In melting (or “ fusion ”), a solid turns into. Condensation is the change of state. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Evaporation is. What Is A Evaporation Phase Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers What Is A Evaporation Phase Change Define melting, freezing, vaporization, condensation, sublimation, and deposition. Solve calorimetry problems involving phase changes. Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water vapor,. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Instead, the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. In melting (or “ fusion ”), a solid turns into. Phase transitions play an important theoretical and practical role in the. What Is A Evaporation Phase Change.
From www.dreamstime.com
Evaporation and Boiling Point of Water . Vector Stock Vector What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Define melting, freezing, vaporization, condensation, sublimation, and deposition. Condensation is the change of state. Phase transitions play an important theoretical and practical role in the study of heat flow. In melting (or “ fusion ”), a solid turns into.. What Is A Evaporation Phase Change.
From www.slideserve.com
PPT Chapter 8 Heat Transfer & Change of Phase PowerPoint Presentation What Is A Evaporation Phase Change Evaporation, process by which an element or compound transitions from its liquid state to its gaseous state below the temperature at. Phase transitions play an important theoretical and practical role in the study of heat flow. For starters, evaporation is the process by which liquid water molecules break the bonds with neighboring molecules and escape into the air as water. What Is A Evaporation Phase Change.