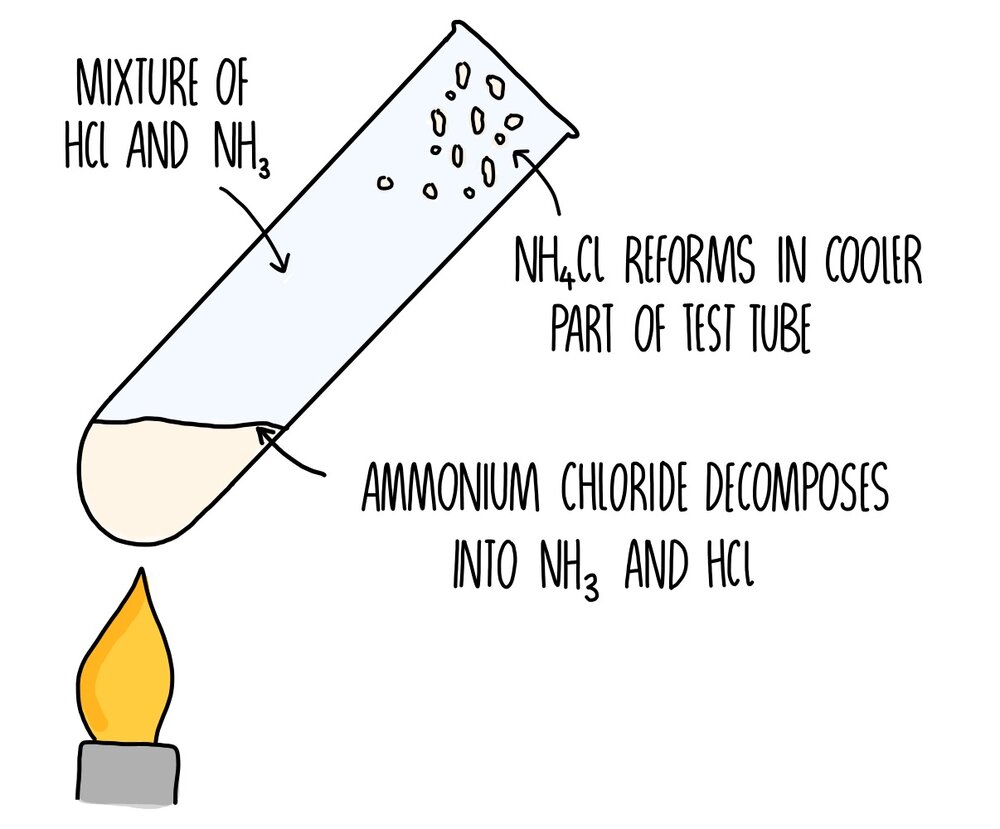
Zinc Hydroxide Reacts With Ammonium Chloride . Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Balance any equation or reaction using this chemical equation balancer!. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Enter an equation of an ionic chemical equation and press the balance button. So, you see white precipitate. Ammonia forms ammonium salts when it reacts with acids. Cu (no3)2 + kno3 = cuno3 + k (no3)2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. For instance, ammonia reacts with hydrochloric acid to make ammonium. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white.
from www.thesciencehive.co.uk
For instance, ammonia reacts with hydrochloric acid to make ammonium. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. Enter an equation of an ionic chemical equation and press the balance button. Ammonia forms ammonium salts when it reacts with acids. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Cu (no3)2 + kno3 = cuno3 + k (no3)2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. So, you see white precipitate.
Reversible Reactions and Equilibria (GCSE) — the science sauce
Zinc Hydroxide Reacts With Ammonium Chloride \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Enter an equation of an ionic chemical equation and press the balance button. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. For instance, ammonia reacts with hydrochloric acid to make ammonium. Balance any equation or reaction using this chemical equation balancer!. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Cu (no3)2 + kno3 = cuno3 + k (no3)2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. So, you see white precipitate. Ammonia forms ammonium salts when it reacts with acids. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl.
From www.numerade.com
SOLVED Write an unbalanced equation to represent each of the following Zinc Hydroxide Reacts With Ammonium Chloride The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. So, you see white precipitate. Enter an equation of an ionic chemical equation and press the balance button. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.numerade.com
SOLVED Part 3C Writing Balanced Chemical Equations Write a balanced Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. Cu (no3)2 + kno3 = cuno3 + k (no3)2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. For instance, ammonia reacts with hydrochloric acid to make ammonium. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Zinc(ii) ion reacts with aqueous ammonia to. Zinc Hydroxide Reacts With Ammonium Chloride.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Hydroxide Reacts With Ammonium Chloride Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. For instance, ammonia reacts with hydrochloric acid to make ammonium. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Balance any equation or reaction using this chemical equation balancer!. So, you see white. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Cu (no3)2 + kno3 = cuno3 + k (no3)2. Ammonia forms ammonium salts when it reacts with acids. For instance, ammonia. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Reaction of zinc sulphate (ZnSO4) with Ammonium hydroxide (NH4OH) YouTube Zinc Hydroxide Reacts With Ammonium Chloride For instance, ammonia reacts with hydrochloric acid to make ammonium. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. So, you see white precipitate. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. Ammonia forms ammonium salts when it. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Hydroxide Reacts With Ammonium Chloride Cu (no3)2 + kno3 = cuno3 + k (no3)2. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. So, you see white precipitate. Enter an equation of an. Zinc Hydroxide Reacts With Ammonium Chloride.
From asideload7.gitlab.io
Outstanding Reaction Between Ammonia And Hydrogen Chloride Zinc Hydroxide Reacts With Ammonium Chloride \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. For instance, ammonia reacts with hydrochloric acid to make ammonium. Ammonia forms ammonium salts when it reacts with acids. Balance any equation or reaction using this chemical equation balancer!. As a result of the reaction of zinc chloride (zncl 2). Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Reaction between calcium hydroxide and ammonium chloride Ca(OH)2 Zinc Hydroxide Reacts With Ammonium Chloride For instance, ammonia reacts with hydrochloric acid to make ammonium. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Cu (no3)2 + kno3 = cuno3 + k (no3)2. Balance any equation or reaction using this chemical equation balancer!. \[\ce{zn^{2+}(aq). Zinc Hydroxide Reacts With Ammonium Chloride.
From www.nagwa.com
Question Video Selecting the Correction Equation for the Reversible Zinc Hydroxide Reacts With Ammonium Chloride Enter an equation of an ionic chemical equation and press the balance button. Ammonia forms ammonium salts when it reacts with acids. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As a result of the reaction of zinc chloride (zncl 2) and ammonium. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc Hydroxide Reacts With Ammonium Chloride Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Ammonia forms ammonium salts when it reacts with acids. Balance any equation or reaction using this chemical equation balancer!. Enter an equation of an ionic chemical equation and press the balance button. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh). Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Hydroxide Reacts With Ammonium Chloride Enter an equation of an ionic chemical equation and press the balance button. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. The zinc ion will react with the ammonia solution to form zinc. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.chegg.com
Solved 5. Zinc metal reacts with hydrochloric acid to Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Balance any equation or reaction using this chemical equation balancer!. For instance, ammonia reacts with hydrochloric acid to make ammonium. The zinc ion will react with the ammonia solution to form zinc hydroxide which is. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Ammonium Chloride Acid Base Reaction chemistry chemicalreaction Zinc Hydroxide Reacts With Ammonium Chloride As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. Zinc(ii) ion reacts with. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Hydroxide Reacts With Ammonium Chloride Cu (no3)2 + kno3 = cuno3 + k (no3)2. So, you see white precipitate. Ammonia forms ammonium salts when it reacts with acids. For instance, ammonia reacts with hydrochloric acid to make ammonium. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. The zinc ion will react with the ammonia. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.researchgate.net
(PDF) Reaction of zinc oxide with ammonium chloride Zinc Hydroxide Reacts With Ammonium Chloride Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Ammonia forms ammonium salts when it reacts with acids. Enter an equation of an ionic chemical equation and press the balance button. So, you see white precipitate. Cu (no3)2 + kno3 = cuno3 + k (no3)2. As a result of the reaction of zinc chloride (zncl 2) and ammonium. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white.. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.mdpi.com
Metals Free FullText Hydrometallurgical Process for Zinc Recovery Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. The zinc ion. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.numerade.com
SOLVED Zinc metal reacts with concentrated nitric acid to give zinc Zinc Hydroxide Reacts With Ammonium Chloride Cu (no3)2 + kno3 = cuno3 + k (no3)2. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Enter an equation of an ionic chemical equation and press the balance button. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Zinc(ii) ion reacts with aqueous ammonia to precipitate. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.slideserve.com
PPT Na 3 PO 4 + 3 KOH â 3 NaOH + K 3 PO 4 Double Displacement Zinc Hydroxide Reacts With Ammonium Chloride Enter an equation of an ionic chemical equation and press the balance button. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Ammonia forms ammonium salts when it reacts with acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Cu (no3)2 + kno3 = cuno3 + k (no3)2. So, you see white precipitate. Zinc(ii) ion. Zinc Hydroxide Reacts With Ammonium Chloride.
From byjus.com
The reduction of nitrobenzene in the presence of Zn/Ammonium chloride Zinc Hydroxide Reacts With Ammonium Chloride Balance any equation or reaction using this chemical equation balancer!. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. So, you see white precipitate. For instance, ammonia reacts with hydrochloric acid to make ammonium.. Zinc Hydroxide Reacts With Ammonium Chloride.
From discover.hubpages.com
The Difference in the Chemistry of Hydrolysis & Hydration. HubPages Zinc Hydroxide Reacts With Ammonium Chloride \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. So, you see white precipitate. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zncl 2 +. Zinc Hydroxide Reacts With Ammonium Chloride.
From tyrelldesnhlawson.blogspot.com
Ammonium Chloride and Sodium Hydroxide Net Ionic Equation Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. For instance, ammonia reacts with hydrochloric acid. Zinc Hydroxide Reacts With Ammonium Chloride.
From brainly.in
9(a)Write the chemical equation for the reaction of zinc metal with(i Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Zncl 2 + 2nh 4 oh → zn (oh) 2 +. Zinc Hydroxide Reacts With Ammonium Chloride.
From zavierfersprince.blogspot.com
Ammonia Nh3 Reacts With Hydrogen Chloride to Form Ammonium Chloride Zinc Hydroxide Reacts With Ammonium Chloride As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. So, you see white precipitate. For instance, ammonia reacts with hydrochloric acid to make ammonium. Enter an equation of an ionic chemical equation and press. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Hydroxide Reacts With Ammonium Chloride Cu (no3)2 + kno3 = cuno3 + k (no3)2. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. So, you see white precipitate. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. The zinc ion will react with the ammonia solution to. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.researchgate.net
Reagents and conditions. (a) Ammonium chloride, zinc acetate, sodium Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer!. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zncl 2 + 2nh 4. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.thesciencehive.co.uk
Reversible Reactions and Equilibria (GCSE) — the science sauce Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Balance any equation or reaction using this chemical equation balancer!. Cu (no3)2 + kno3 = cuno3 + k (no3)2. For instance, ammonia reacts with hydrochloric. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.youtube.com
Ammonium Chloride synthesis and properties YouTube Zinc Hydroxide Reacts With Ammonium Chloride Enter an equation of an ionic chemical equation and press the balance button. Cu (no3)2 + kno3 = cuno3 + k (no3)2. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Ammonia forms ammonium salts when it reacts with acids. For instance, ammonia reacts. Zinc Hydroxide Reacts With Ammonium Chloride.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Hydroxide Reacts With Ammonium Chloride So, you see white precipitate. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. For instance, ammonia reacts with hydrochloric acid to make ammonium. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Enter an equation of an ionic chemical equation and press. Zinc Hydroxide Reacts With Ammonium Chloride.
From byjus.com
A compound is heated with zinc dust and ammonium chloride followed by Zinc Hydroxide Reacts With Ammonium Chloride For instance, ammonia reacts with hydrochloric acid to make ammonium. Enter an equation of an ionic chemical equation and press the balance button. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Cu (no3)2 + kno3 = cuno3 + k (no3)2. So, you see white precipitate. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. Ammonia forms ammonium. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.researchgate.net
(PDF) Review of Ammonium ChlorideWater Solution Properties Zinc Hydroxide Reacts With Ammonium Chloride Cu (no3)2 + kno3 = cuno3 + k (no3)2. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Ammonia. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.numerade.com
SOLVEDThe reaction of a drycell battery may be represented as follows Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. Zncl 2 + 2nh 4 oh → zn (oh) 2 + 2nh 4 cl. For instance, ammonia reacts with hydrochloric acid to make ammonium. The zinc ion will react with the ammonia solution to form zinc hydroxide which is white. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Zinc Hydroxide Reacts With Ammonium Chloride Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Ammonia forms ammonium salts when it reacts with acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Zncl 2 + 2nh 4 oh. Zinc Hydroxide Reacts With Ammonium Chloride.
From byjus.com
Balance the reaction of barium hydroxide and ammonium chloride by the Zinc Hydroxide Reacts With Ammonium Chloride Balance any equation or reaction using this chemical equation balancer!. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Ammonia forms ammonium salts when it reacts with acids. Cu (no3)2 + kno3 = cuno3 + k (no3)2. Enter an equation of an ionic chemical equation and press the balance button.. Zinc Hydroxide Reacts With Ammonium Chloride.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Zinc Hydroxide Reacts With Ammonium Chloride Ammonia forms ammonium salts when it reacts with acids. As a result of the reaction of zinc chloride (zncl 2) and ammonium hydroxide (nh 4 oh) produces zinc. Balance any equation or reaction using this chemical equation balancer!. Cu (no3)2 + kno3 = cuno3 + k (no3)2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to. Zinc Hydroxide Reacts With Ammonium Chloride.