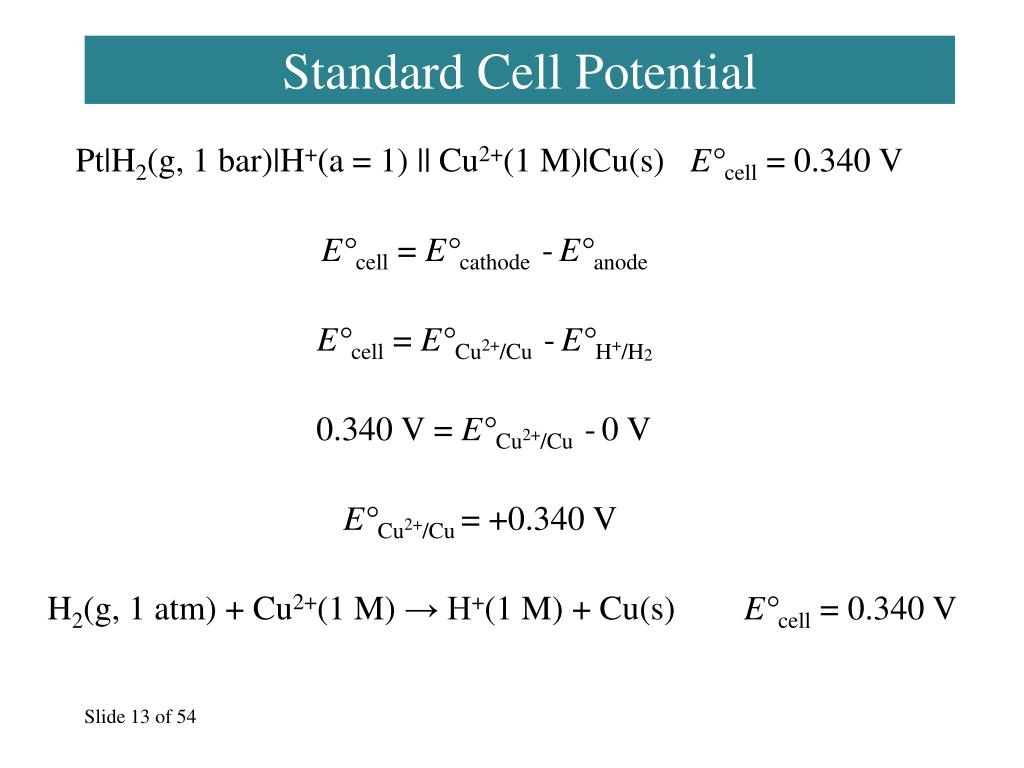
Standard Cell Potential Eo . The difference between the two half cells is calculated using the equation below: Eº cell is the standard state cell potential, which means that the value was determined under standard states. Is the standard cell potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Difference between e cell and eº cell. E 0 cell = e 0 red, cathode = e 0 red, anode. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states:
from www.slideserve.com
Eº cell is the standard state cell potential, which means that the value was determined under standard states. E 0 cell = e 0 red, cathode = e 0 red, anode. Is the standard cell potential The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Interpret electrode potentials in terms of relative oxidant and reductant. The difference between the two half cells is calculated using the equation below: Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes.
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free
Standard Cell Potential Eo E 0 cell = e 0 red, cathode = e 0 red, anode. Difference between e cell and eº cell. Interpret electrode potentials in terms of relative oxidant and reductant. Eº cell is the standard state cell potential, which means that the value was determined under standard states. E 0 cell = e 0 red, cathode = e 0 red, anode. Is the standard cell potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Describe and relate the definitions of electrode and cell potentials. The difference between the two half cells is calculated using the equation below: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states:
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Eo The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Difference between e cell and eº cell. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The difference between the two half cells is calculated using the equation below:. Standard Cell Potential Eo.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Eo E 0 cell = e 0 red, cathode = e 0 red, anode. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Difference between e cell and eº cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard. Standard Cell Potential Eo.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Eo E 0 cell = e 0 red, cathode = e 0 red, anode. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage. Standard Cell Potential Eo.
From www.numerade.com
SOLVED Question 1 of 30 Submit What is the standard cell potential for Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Interpret electrode potentials in terms of relative oxidant and reductant. Eº cell is the standard state cell potential, which means that the value was. Standard Cell Potential Eo.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. E 0 cell = e 0 red, cathode = e 0 red, anode. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the. Standard Cell Potential Eo.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Cell Potential Eo Difference between e cell and eº cell. Eº cell is the standard state cell potential, which means that the value was determined under standard states. E 0 cell = e 0 red, cathode = e 0 red, anode. Is the standard cell potential The standard cell potential (e°cell) is measured at 298 k and all components in their standard states:. Standard Cell Potential Eo.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Eo Describe and relate the definitions of electrode and cell potentials. The difference between the two half cells is calculated using the equation below: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant. Is the standard cell potential The standard cell potential (e°cell) is measured at. Standard Cell Potential Eo.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Standard Cell Potential Eo The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Eº cell is the standard state cell potential, which means that the value was determined under standard states. The standard cell potential. Standard Cell Potential Eo.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Eo Is the standard cell potential E 0 cell = e 0 red, cathode = e 0 red, anode. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Difference between e cell and eº cell. The standard cell potential (e 0 cell) is the difference between the two electrodes that. Standard Cell Potential Eo.
From www.slideserve.com
PPT Electrochemistry Part III Reduction Potentials PowerPoint Standard Cell Potential Eo 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Is the standard cell potential Eº cell is the standard state cell potential, which means that the value was determined under standard states. E 0 cell = e 0 red, cathode = e 0 red, anode. Difference between e cell and eº cell. Describe and relate. Standard Cell Potential Eo.
From slideplayer.com
ELECTROCHEMICAL CELLS Chapter 20 D8 C20 ppt download Standard Cell Potential Eo E 0 cell = e 0 red, cathode = e 0 red, anode. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The standard cell potential (e 0 cell) is the difference between the two electrodes. Standard Cell Potential Eo.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: E 0 cell = e 0 red, cathode = e 0 red, anode. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. 1 atm for gases, 1 m for solutions, and. Standard Cell Potential Eo.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Standard Cell Potential Eo 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. E 0 cell = e 0 red, cathode = e 0 red, anode. Interpret electrode potentials in terms of relative oxidant and reductant. Is the standard cell potential The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The. Standard Cell Potential Eo.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The difference between the two half cells is calculated using the equation below: E 0 cell = e 0 red, cathode = e 0 red, anode. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of. Standard Cell Potential Eo.
From app.pandai.org
Standard Electrode Potential Standard Cell Potential Eo Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Is the standard cell potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Interpret electrode potentials in terms of relative oxidant and. Standard Cell Potential Eo.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6601383 Standard Cell Potential Eo The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Describe and relate the definitions of electrode and cell potentials. The difference between the two half cells is calculated using the equation below: Is the standard cell potential Difference between e cell and eº cell. The standard cell potential. Standard Cell Potential Eo.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Eo The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. The difference between the two half cells is calculated using the equation below: Interpret electrode potentials in terms of relative oxidant and reductant. Is the standard cell potential The standard cell potential (e 0 cell) is the difference between the two. Standard Cell Potential Eo.
From www.youtube.com
2039 Calculating Standard Cell Potentials YouTube Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. Eº cell is the standard state cell potential, which means that the value was determined under standard states. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. E. Standard Cell Potential Eo.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Cell Potential Eo The difference between the two half cells is calculated using the equation below: E 0 cell = e 0 red, cathode = e 0 red, anode. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The standard cell. Standard Cell Potential Eo.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential Eo Describe and relate the definitions of electrode and cell potentials. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. Difference between e cell and eº cell. Eº cell is the standard state cell potential, which means that the value was determined under standard states. The difference between the two half. Standard Cell Potential Eo.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potential Eo Eº cell is the standard state cell potential, which means that the value was determined under standard states. Is the standard cell potential Interpret electrode potentials in terms of relative oxidant and reductant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. E 0 cell = e 0 red, cathode = e 0 red, anode.. Standard Cell Potential Eo.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Eo Difference between e cell and eº cell. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. E 0 cell = e 0 red, cathode = e 0 red, anode. Describe and relate the definitions of electrode and cell potentials. The difference between the two half cells is calculated. Standard Cell Potential Eo.
From www.slideserve.com
PPT Calculating the Cell Potential PowerPoint Presentation, free Standard Cell Potential Eo Is the standard cell potential 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Difference between e cell and eº cell. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials. Standard Cell Potential Eo.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2133149 Standard Cell Potential Eo The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The difference between the two half cells is calculated using the equation below: Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential. Standard Cell Potential Eo.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Eo The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Describe and relate the definitions of electrode and cell potentials. The difference between the two half cells is calculated using the equation below: E 0 cell = e 0 red, cathode = e 0 red, anode. Eº cell is. Standard Cell Potential Eo.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Difference between e cell and eº cell. The difference between the two half cells is calculated using the equation below: Is the standard cell potential The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that. Standard Cell Potential Eo.
From www.chegg.com
Solved 17. Using standard reduction potentials (on the next Standard Cell Potential Eo Eº cell is the standard state cell potential, which means that the value was determined under standard states. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: E 0 cell = e 0 red, cathode = e 0. Standard Cell Potential Eo.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Eo Is the standard cell potential The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The difference between the two half cells is calculated using the equation below: Eº cell is the standard state cell potential, which means that the value was determined under standard states. E 0 cell = e 0 red,. Standard Cell Potential Eo.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential Eo The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. E 0 cell = e 0 red, cathode = e 0 red, anode. Difference between e cell and eº cell. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell.. Standard Cell Potential Eo.
From quizdbcornwallis.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potential Eo E 0 cell = e 0 red, cathode = e 0 red, anode. The difference between the two half cells is calculated using the equation below: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Difference between e cell and eº cell. The standard cell potential (e°cell) is measured at 298 k and all components. Standard Cell Potential Eo.
From www.chegg.com
Solved Calculate the standard cell potential for each of the Standard Cell Potential Eo Describe and relate the definitions of electrode and cell potentials. Eº cell is the standard state cell potential, which means that the value was determined under standard states. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (\(e^o_{cell}\)) is the difference of the two electrodes, which forms the voltage of that cell. 1 atm for. Standard Cell Potential Eo.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Eo The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. Eº cell is the standard state cell potential, which means that the value was determined under standard states. Is the standard cell potential The standard cell potential (e°cell) is measured at 298 k and all components in their standard. Standard Cell Potential Eo.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Eo The difference between the two half cells is calculated using the equation below: Eº cell is the standard state cell potential, which means that the value was determined under standard states. Difference between e cell and eº cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential (\(e^o_{cell}\)). Standard Cell Potential Eo.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Standard Cell Potential Eo Difference between e cell and eº cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The difference between the two half cells is calculated using the equation. Standard Cell Potential Eo.
From slideplayer.com
Review Unit 8 (Chp 20) Electrochemistry ppt download Standard Cell Potential Eo The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: E 0 cell = e 0 red, cathode = e 0 red, anode. The difference between the two half cells is calculated using the equation below: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Eº cell is. Standard Cell Potential Eo.