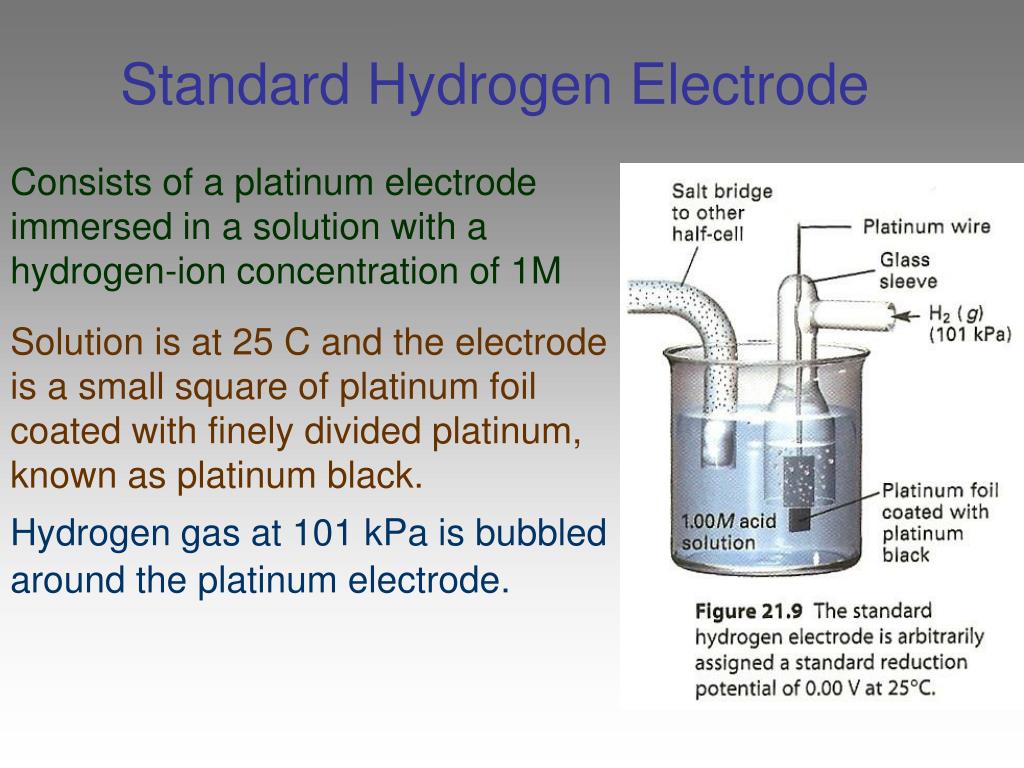
Standard Hydrogen Electrode Ppt . The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode.
from www.slideserve.com
The structure of the standard hydrogen electrode is described. Potential of a cell composed of a working electrode (1 m. What is a standard hydrogen electrode? This is because it acts as a. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k.
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free
Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Potential of a cell composed of a working electrode (1 m. What is a standard hydrogen electrode?
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. This is because it acts as a. Examples of using. Standard Hydrogen Electrode Ppt.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Potential of a cell composed of a working electrode (1 m. Examples of using the standard hydrogen electrode to determine reduction potentials are given. An ideal reference electrode has a reproducible and stable potential that is. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. This is because it acts as a. Examples of using the standard hydrogen electrode to determine reduction potentials are given. What is a standard hydrogen electrode? An. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt What is a standard hydrogen electrode? This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. The structure of. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. This is because it acts as a. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or. Standard Hydrogen Electrode Ppt.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Standard Hydrogen Electrode Ppt Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Potential of a cell composed of a working electrode (1 m. This is because. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Potential of a cell composed of a working electrode (1 m. This is because it acts as a. An ideal reference electrode has. Standard Hydrogen Electrode Ppt.
From dohsdocfeco.blob.core.windows.net
Facts About Standard Hydrogen Electrode at Troy Williams blog Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode in order to measure the potential of an electrode, it. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine reduction potentials are given. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Hydrogen Electrode Ppt Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a. Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The standard hydrogen electrode is. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. An ideal reference electrode has a reproducible and stable potential that. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Standard Hydrogen Electrode Ppt This is because it acts as a. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. What is a standard hydrogen electrode? Examples of using the standard hydrogen electrode to determine reduction potentials are given. An ideal reference electrode has a reproducible and stable potential that is not affected. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. Potential of a cell composed of a working electrode (1 m. What is a standard hydrogen electrode? This is because it acts as a. An ideal reference electrode has a reproducible and stable potential that is not affected by small. Standard Hydrogen Electrode Ppt.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Ppt The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. This is because it acts as a. The structure of the standard hydrogen electrode is described. Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not. Standard Hydrogen Electrode Ppt.
From slideplayer.com
Electrochemistry. ppt download Standard Hydrogen Electrode Ppt Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Ppt Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Potential of a cell composed of a working electrode (1 m. The standard hydrogen electrode in order to measure the potential of an. Standard Hydrogen Electrode Ppt.
From dokumen.tips
(PPT) STANDARD ELECTRODE POTENTIALS. THE STANDARD HYDROGEN ELECTRODE In Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode? This is because it acts as a. An ideal reference electrode has a reproducible and stable potential that is not affected. Standard Hydrogen Electrode Ppt.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Standard Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. The standard hydrogen electrode is often abbreviated. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Standard Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. Potential of a cell composed of a working electrode (1 m. What is a standard hydrogen electrode? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt What is a standard hydrogen electrode? Examples of using the standard hydrogen electrode to determine reduction potentials are given. This is because it acts as a. Potential of a cell composed of a working electrode (1 m. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes. Standard Hydrogen Electrode Ppt.
From dokumen.tips
(PPT) Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Ppt This is because it acts as a. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode in order to. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Standard Hydrogen Electrode Ppt Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. Potential of a cell composed of a working electrode (1 m. An ideal reference electrode has a reproducible and stable potential that is not affected by small. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt This is because it acts as a. Potential of a cell composed of a working electrode (1 m. The structure of the standard hydrogen electrode is described. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a standard hydrogen electrode? The standard hydrogen. Standard Hydrogen Electrode Ppt.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Ppt Potential of a cell composed of a working electrode (1 m. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The standard hydrogen electrode in order. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT REDOX EQM Standard Hydrogen Electrode and Measurement of Standard Hydrogen Electrode Ppt This is because it acts as a. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Standard Hydrogen Electrode Ppt.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Ppt The structure of the standard hydrogen electrode is described. This is because it acts as a. Potential of a cell composed of a working electrode (1 m. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. What is a standard hydrogen electrode? Examples of using the standard hydrogen electrode. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Standard Hydrogen Electrode Ppt What is a standard hydrogen electrode? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The standard hydrogen electrode in order. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT REDOX EQM Standard Hydrogen Electrode and Measurement of Standard Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. This is because it acts as a. Potential of a cell composed of a working electrode (1 m. What is a standard hydrogen electrode? The standard hydrogen electrode in order to measure the potential of an. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Ppt This is because it acts as a. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? Potential of a cell composed of a working electrode (1 m. Examples of using the standard hydrogen electrode to determine reduction potentials are. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Ppt The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The structure of the standard hydrogen electrode is described. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a. Standard Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID820788 Standard Hydrogen Electrode Ppt What is a standard hydrogen electrode? This is because it acts as a. Potential of a cell composed of a working electrode (1 m. The standard hydrogen electrode in order to measure the potential of an electrode, it is compared to a reference electrode. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared. Standard Hydrogen Electrode Ppt.