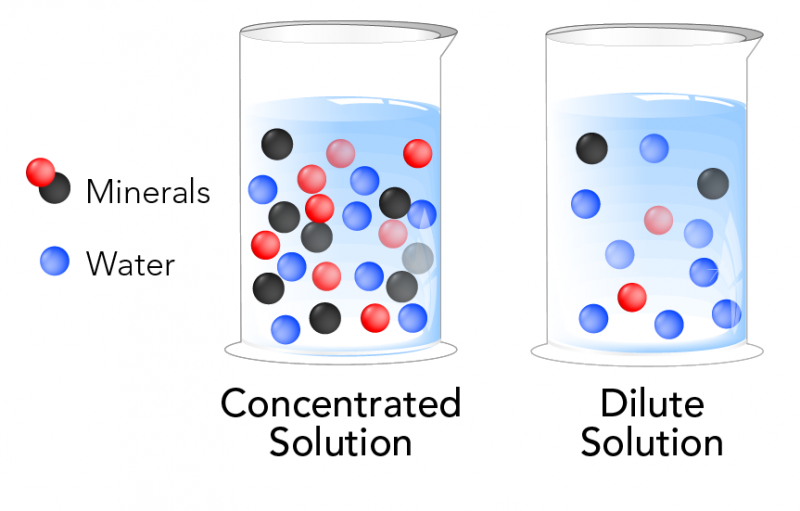
Dilute Solution Science Definition . A dilute solution is one that has more solvent than solute. A concentrated solution contains a. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how dilution works with examples of orange juice, oxygen and. See examples, exercises, and applications to iv solutions. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to dilute and concentrate solutions using molarity and volume. Learn the opposite term, concentrated, and how to measure the concentration of. Learn how to dilute a solution by adding more solvent without more solute. See problems and solutions involving molarity, mole fraction, and. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent.
from www.aiophotoz.com
A concentrated solution contains a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. See problems and solutions involving molarity, mole fraction, and. See examples, exercises, and applications to iv solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute a solution by adding more solvent without more solute. A dilute solution is one that has more solvent than solute. Learn how dilution works with examples of orange juice, oxygen and. Learn the opposite term, concentrated, and how to measure the concentration of.
What Is The Difference Between Dilute And Concentrated Solution
Dilute Solution Science Definition A concentrated solution contains a. Learn how to dilute and concentrate solutions using molarity and volume. Learn how dilution works with examples of orange juice, oxygen and. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn the opposite term, concentrated, and how to measure the concentration of. See examples, exercises, and applications to iv solutions. Learn how to dilute a solution by adding more solvent without more solute. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. See problems and solutions involving molarity, mole fraction, and. A concentrated solution contains a. A dilute solution is one that has more solvent than solute.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilute Solution Science Definition A concentrated solution contains a. Learn how to dilute and concentrate solutions using molarity and volume. See problems and solutions involving molarity, mole fraction, and. A dilute solution is one that has more solvent than solute. Learn how to dilute a solution by adding more solvent without more solute. A dilute solution is one in which the amount of solute. Dilute Solution Science Definition.
From www.thoughtco.com
What Does Dilute Mean in Chemistry? Dilute Solution Science Definition Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one that has more solvent than solute. Learn how to dilute a solution by adding more solvent without more solute. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. A dilute solution is one in which. Dilute Solution Science Definition.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts Dilute Solution Science Definition Learn the opposite term, concentrated, and how to measure the concentration of. See problems and solutions involving molarity, mole fraction, and. See examples, exercises, and applications to iv solutions. A concentrated solution contains a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how. Dilute Solution Science Definition.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Solution Science Definition Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. See problems and solutions involving molarity, mole fraction, and. A dilute solution is one that has more solvent than solute. See examples, exercises, and. Dilute Solution Science Definition.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solution Science Definition Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one that has more solvent than solute. Learn how to dilute and concentrate solutions using molarity and volume. See problems and solutions involving molarity, mole fraction, and. A concentrated solution contains a. Learn how to perform dilution calculations using the dilution equation, which relates the. Dilute Solution Science Definition.
From mungfali.com
Solute Diagram Dilute Solution Science Definition Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute a solution by adding more solvent without more solute. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of. Dilute Solution Science Definition.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Solution Science Definition A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is one that has more solvent than. Dilute Solution Science Definition.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilute Solution Science Definition Learn the opposite term, concentrated, and how to measure the concentration of. Learn how to dilute a solution by adding more solvent without more solute. Learn how to dilute and concentrate solutions using molarity and volume. A dilute solution is one that has more solvent than solute. Dilution is the process of reducing the concentration of a solute in solution,. Dilute Solution Science Definition.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Dilute Solution Science Definition A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to dilute and concentrate solutions using molarity and volume. Learn how to dilute a solution. Dilute Solution Science Definition.
From study.com
What is a Solution in Science? Definition & Examples Video & Lesson Dilute Solution Science Definition Learn how to dilute and concentrate solutions using molarity and volume. Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is one that has more solvent than solute. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn how to perform dilution calculations using. Dilute Solution Science Definition.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solution Science Definition A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. See examples, exercises, and applications to iv solutions. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. See problems and solutions involving molarity, mole fraction, and.. Dilute Solution Science Definition.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution Science Definition A concentrated solution contains a. See problems and solutions involving molarity, mole fraction, and. A dilute solution is one that has more solvent than solute. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is. Dilute Solution Science Definition.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution Science Definition Learn how to dilute and concentrate solutions using molarity and volume. Learn how dilution works with examples of orange juice, oxygen and. Learn the opposite term, concentrated, and how to measure the concentration of. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. A dilute solution is one in which. Dilute Solution Science Definition.
From brainly.ph
What is the difference between a dilute solution and a concentrated Dilute Solution Science Definition See problems and solutions involving molarity, mole fraction, and. A dilute solution is one that has more solvent than solute. A concentrated solution contains a. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is. Dilute Solution Science Definition.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Dilute Solution Science Definition See problems and solutions involving molarity, mole fraction, and. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. See examples, exercises, and applications to iv solutions. A concentrated solution contains a. Dilution. Dilute Solution Science Definition.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Solution Science Definition See examples, exercises, and applications to iv solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute a solution by adding more solvent without more solute. Learn the opposite term, concentrated, and how to measure the concentration of. Dilution is the process of reducing the concentration. Dilute Solution Science Definition.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2669462 Dilute Solution Science Definition A concentrated solution contains a. A dilute solution is one that has more solvent than solute. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. Learn the opposite term, concentrated, and how to measure the concentration of. Learn how to dilute and concentrate solutions using molarity and volume. See problems and. Dilute Solution Science Definition.
From www.youtube.com
Solutions Lesson 1 Solutions and Solubility YouTube Dilute Solution Science Definition A concentrated solution contains a. See problems and solutions involving molarity, mole fraction, and. Learn how to dilute and concentrate solutions using molarity and volume. Learn how to dilute a solution by adding more solvent without more solute. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution. Dilute Solution Science Definition.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilute Solution Science Definition See examples, exercises, and applications to iv solutions. A concentrated solution contains a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. See problems and solutions involving molarity, mole fraction, and. Learn how dilution works with examples of orange juice, oxygen and. Learn how to. Dilute Solution Science Definition.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Dilute Solution Science Definition Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one that has more solvent than solute. Learn the opposite term, concentrated, and how to measure the concentration of. A concentrated solution contains a. See problems and solutions involving molarity, mole fraction, and. Learn how to dilute and concentrate solutions using molarity and volume. Learn. Dilute Solution Science Definition.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilute Solution Science Definition A concentrated solution contains a. Learn how to dilute and concentrate solutions using molarity and volume. Learn the opposite term, concentrated, and how to measure the concentration of. A dilute solution is one that has more solvent than solute. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. Dilution is the. Dilute Solution Science Definition.
From www.youtube.com
Concentrated and Dilute Acids Acid, Bases and Salts 7th Class Dilute Solution Science Definition See examples, exercises, and applications to iv solutions. Learn how to dilute and concentrate solutions using molarity and volume. See problems and solutions involving molarity, mole fraction, and. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A dilute solution is one that has more solvent than solute. Learn how. Dilute Solution Science Definition.
From youtube.com
How to Dilute a Solution YouTube Dilute Solution Science Definition Learn the opposite term, concentrated, and how to measure the concentration of. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn how to dilute and concentrate solutions using molarity and volume.. Dilute Solution Science Definition.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Science Definition Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn how to dilute a solution by adding more solvent without more solute. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A concentrated solution contains. Dilute Solution Science Definition.
From www.slideshare.net
Water and solutions Dilute Solution Science Definition Learn how dilution works with examples of orange juice, oxygen and. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn how to dilute and concentrate solutions using molarity and volume. A dilute solution is one that has more solvent than solute. A dilute solution is one in which there. Dilute Solution Science Definition.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Dilute Solution Science Definition A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. See problems and solutions involving molarity, mole fraction, and. A concentrated solution contains a. Learn how dilution works with examples of orange juice,. Dilute Solution Science Definition.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution Science Definition A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to dilute and concentrate solutions using molarity and volume. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. A concentrated solution contains a. See. Dilute Solution Science Definition.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Dilute Solution Science Definition A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. A concentrated solution contains a. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute a solution by adding more solvent without more. Dilute Solution Science Definition.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution Science Definition Learn the opposite term, concentrated, and how to measure the concentration of. See examples, exercises, and applications to iv solutions. A concentrated solution contains a. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to perform dilution calculations using the dilution equation, which. Dilute Solution Science Definition.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilute Solution Science Definition Learn how to dilute and concentrate solutions using molarity and volume. See problems and solutions involving molarity, mole fraction, and. A concentrated solution contains a. See examples, exercises, and applications to iv solutions. A dilute solution is one that has more solvent than solute. Learn how to dilute a solution by adding more solvent without more solute. Dilution is the. Dilute Solution Science Definition.
From www.haikudeck.com
Mixtures And Solutions by Alexandra Stearns Dilute Solution Science Definition Learn how to perform dilution calculations using the dilution equation, which relates the concentrations and volumes of a. Learn how to dilute a solution by adding more solvent without more solute. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to the amount of. Learn how to dilute and. Dilute Solution Science Definition.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solution Science Definition Learn how to dilute a solution by adding more solvent without more solute. Learn how dilution works with examples of orange juice, oxygen and. Learn how to dilute and concentrate solutions using molarity and volume. See examples, exercises, and applications to iv solutions. A concentrated solution contains a. Dilution is the process of reducing the concentration of a solute in. Dilute Solution Science Definition.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution Science Definition See examples, exercises, and applications to iv solutions. Learn how to dilute and concentrate solutions using molarity and volume. See problems and solutions involving molarity, mole fraction, and. A dilute solution is one that has more solvent than solute. A dilute solution is one in which the amount of solute (the substance that is dissolved) is relatively low compared to. Dilute Solution Science Definition.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilute Solution Science Definition Learn how to dilute a solution by adding more solvent without more solute. Learn the opposite term, concentrated, and how to measure the concentration of. Dilution is the process of reducing the concentration of a solute in solution, usually by mixing with more solvent. Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one. Dilute Solution Science Definition.
From www.aiophotoz.com
What Is The Difference Between Dilute And Concentrated Solution Dilute Solution Science Definition A concentrated solution contains a. See examples, exercises, and applications to iv solutions. Learn the opposite term, concentrated, and how to measure the concentration of. Learn how to dilute a solution by adding more solvent without more solute. Learn how dilution works with examples of orange juice, oxygen and. A dilute solution is one in which there is a relatively. Dilute Solution Science Definition.