Are Bases Soluble In Bases . Bases often have a bitter taste and are found in foods These are also called alkalis. These are bases which are dissolvable in water. Bases can be either strong or weak, just as acids can. Most bases are minerals that react with acids to form water and salts. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Bases include the oxides, hydroxides and carbonates of. The determining factor for the result is the solubility. An alkaline solution can be formed when a metal oxide is dissolved in water. Aqueous solutions of bases are also electrolytes. However, if a base does dissolve in water, we also call it an. Sodium hydroxide (naoh), potassium hydroxide (koh) Bases bases have properties that mostly contrast with those of acids. Bases can be classified as soluble and insoluble. When a substance is mixed with a solvent, there are several possible results.
from www.slideserve.com
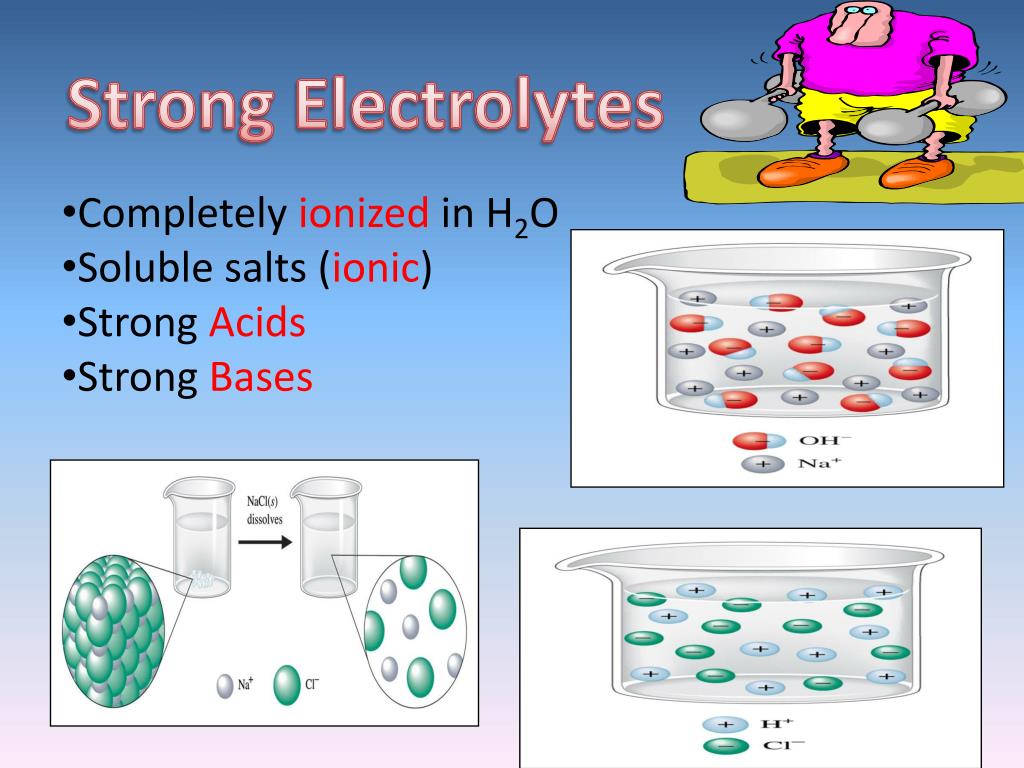
Aqueous solutions of bases are also electrolytes. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases can be either strong or weak, just as acids can. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Bases often have a bitter taste and are found in foods The determining factor for the result is the solubility. Bases include the oxides, hydroxides and carbonates of. Bases can be classified as soluble and insoluble. These are also called alkalis. Sodium hydroxide (naoh), potassium hydroxide (koh)
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free
Are Bases Soluble In Bases Most bases are minerals that react with acids to form water and salts. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases can be classified as soluble and insoluble. Most bases are minerals that react with acids to form water and salts. Aqueous solutions of bases are also electrolytes. Bases bases have properties that mostly contrast with those of acids. When a substance is mixed with a solvent, there are several possible results. However, if a base does dissolve in water, we also call it an. Bases can be either strong or weak, just as acids can. Sodium hydroxide (naoh), potassium hydroxide (koh) These are bases which are dissolvable in water. Bases include the oxides, hydroxides and carbonates of. The determining factor for the result is the solubility. These are also called alkalis. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Bases often have a bitter taste and are found in foods
From brainly.in
name some insoluble bases Brainly.in Are Bases Soluble In Bases The determining factor for the result is the solubility. An alkaline solution can be formed when a metal oxide is dissolved in water. Sodium hydroxide (naoh), potassium hydroxide (koh) These are bases which are dissolvable in water. Bases often have a bitter taste and are found in foods When a substance is mixed with a solvent, there are several possible. Are Bases Soluble In Bases.
From slideplayer.com
Chapter 18 Carboxylic Acids ppt download Are Bases Soluble In Bases Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases often have a bitter taste and are found in foods Bases include the oxides, hydroxides and carbonates of. These are bases which are dissolvable in water. These are also. Are Bases Soluble In Bases.
From askfilo.com
CHAPTER 3A A Acids, Bases and Salts Examples with equationfor the loni.. Are Bases Soluble In Bases Bases bases have properties that mostly contrast with those of acids. Bases include the oxides, hydroxides and carbonates of. However, if a base does dissolve in water, we also call it an. Sodium hydroxide (naoh), potassium hydroxide (koh) Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Bases often have a bitter taste. Are Bases Soluble In Bases.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Are Bases Soluble In Bases Bases bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods Most bases are minerals that react with acids to form water and salts. An alkaline solution can be formed when a metal oxide is dissolved in water. When a substance is mixed with a solvent, there are several. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Topical delivery dosage forms PowerPoint Presentation, free Are Bases Soluble In Bases An alkaline solution can be formed when a metal oxide is dissolved in water. Bases bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods Bases include the oxides, hydroxides and carbonates of. Bases can be either strong or weak, just as acids can. These are also called alkalis.. Are Bases Soluble In Bases.
From www.pinterest.com
Pin on Acids Bases and Solutions Are Bases Soluble In Bases However, if a base does dissolve in water, we also call it an. Sodium hydroxide (naoh), potassium hydroxide (koh) Bases can be classified as soluble and insoluble. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. When a substance is mixed with a solvent, there are several possible results. The determining factor for. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Acids and Bases Properties, Testing, and pH Scale PowerPoint Are Bases Soluble In Bases These are also called alkalis. Bases can be classified as soluble and insoluble. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Sodium hydroxide (naoh), potassium hydroxide (koh) However, if a base does dissolve in water, we also call. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Water, Electrolytes, and Solutions PowerPoint Presentation, free Are Bases Soluble In Bases Bases bases have properties that mostly contrast with those of acids. The determining factor for the result is the solubility. However, if a base does dissolve in water, we also call it an. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases include the oxides, hydroxides and carbonates of. Aqueous solutions of bases are. Are Bases Soluble In Bases.
From wordwall.net
Acids and Alkalis Match up Are Bases Soluble In Bases However, if a base does dissolve in water, we also call it an. Bases can be classified as soluble and insoluble. An alkaline solution can be formed when a metal oxide is dissolved in water. The determining factor for the result is the solubility. Aqueous solutions of bases are also electrolytes. Bases bases have properties that mostly contrast with those. Are Bases Soluble In Bases.
From www.myxxgirl.com
Ppt Acids Bases And Alkalis Powerpoint Presentation Id My XXX Hot Girl Are Bases Soluble In Bases Aqueous solutions of bases are also electrolytes. These are bases which are dissolvable in water. However, if a base does dissolve in water, we also call it an. Bases can be either strong or weak, just as acids can. The determining factor for the result is the solubility. Most bases are minerals that react with acids to form water and. Are Bases Soluble In Bases.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii Are Bases Soluble In Bases Most bases are minerals that react with acids to form water and salts. Bases bases have properties that mostly contrast with those of acids. Sodium hydroxide (naoh), potassium hydroxide (koh) Aqueous solutions of bases are also electrolytes. Bases can be classified as soluble and insoluble. Bases often have a bitter taste and are found in foods An alkaline solution can. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Topical delivery dosage forms PowerPoint Presentation ID300896 Are Bases Soluble In Bases Sodium hydroxide (naoh), potassium hydroxide (koh) Bases can be either strong or weak, just as acids can. The determining factor for the result is the solubility. Bases often have a bitter taste and are found in foods However, if a base does dissolve in water, we also call it an. Bases can be classified as soluble and insoluble. An alkaline. Are Bases Soluble In Bases.
From www.slideserve.com
PPT CHAPTER 10 Reactions in Aqueous Solutions I Acids, Bases & Salts Are Bases Soluble In Bases Bases can be classified as soluble and insoluble. Bases can be either strong or weak, just as acids can. Bases include the oxides, hydroxides and carbonates of. Sodium hydroxide (naoh), potassium hydroxide (koh) These are bases which are dissolvable in water. These are also called alkalis. An alkaline solution can be formed when a metal oxide is dissolved in water.. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Topical delivery dosage forms PowerPoint Presentation ID300896 Are Bases Soluble In Bases The determining factor for the result is the solubility. Bases can be classified as soluble and insoluble. However, if a base does dissolve in water, we also call it an. Bases can be either strong or weak, just as acids can. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. These are bases. Are Bases Soluble In Bases.
From riyakruwellis.blogspot.com
Is Salt Soluble in Water RiyakruwEllis Are Bases Soluble In Bases Bases include the oxides, hydroxides and carbonates of. Bases often have a bitter taste and are found in foods Bases can be classified as soluble and insoluble. Sodium hydroxide (naoh), potassium hydroxide (koh) However, if a base does dissolve in water, we also call it an. Bases can be either strong or weak, just as acids can. Aqueous solutions of. Are Bases Soluble In Bases.
From www.youtube.com
Neutralisation involving sparingly soluble bases // HSC Chemistry YouTube Are Bases Soluble In Bases However, if a base does dissolve in water, we also call it an. Bases often have a bitter taste and are found in foods When a substance is mixed with a solvent, there are several possible results. An alkaline solution can be formed when a metal oxide is dissolved in water. These are bases which are dissolvable in water. Sodium. Are Bases Soluble In Bases.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Are Bases Soluble In Bases Most bases are minerals that react with acids to form water and salts. These are also called alkalis. The determining factor for the result is the solubility. Bases include the oxides, hydroxides and carbonates of. Bases bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods Bases which are. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download Are Bases Soluble In Bases The determining factor for the result is the solubility. Bases bases have properties that mostly contrast with those of acids. An alkaline solution can be formed when a metal oxide is dissolved in water. Sodium hydroxide (naoh), potassium hydroxide (koh) Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just as acids can. Bases which. Are Bases Soluble In Bases.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Are Bases Soluble In Bases An alkaline solution can be formed when a metal oxide is dissolved in water. Bases often have a bitter taste and are found in foods However, if a base does dissolve in water, we also call it an. Most bases are minerals that react with acids to form water and salts. Bases can be classified as soluble and insoluble. Bases. Are Bases Soluble In Bases.
From slideplayer.com
Properties of Solutions and Solubility ppt download Are Bases Soluble In Bases The determining factor for the result is the solubility. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Sodium hydroxide (naoh), potassium hydroxide (koh) Bases bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods Most bases are minerals that react with. Are Bases Soluble In Bases.
From quinntewallace.blogspot.com
Soluble Salts List QuinnteWallace Are Bases Soluble In Bases These are bases which are dissolvable in water. Bases include the oxides, hydroxides and carbonates of. When a substance is mixed with a solvent, there are several possible results. However, if a base does dissolve in water, we also call it an. Bases can be either strong or weak, just as acids can. Bases bases have properties that mostly contrast. Are Bases Soluble In Bases.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Are Bases Soluble In Bases An alkaline solution can be formed when a metal oxide is dissolved in water. These are bases which are dissolvable in water. Most bases are minerals that react with acids to form water and salts. However, if a base does dissolve in water, we also call it an. These are also called alkalis. Bases include the oxides, hydroxides and carbonates. Are Bases Soluble In Bases.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Are Bases Soluble In Bases Bases can be either strong or weak, just as acids can. Bases can be classified as soluble and insoluble. When a substance is mixed with a solvent, there are several possible results. An alkaline solution can be formed when a metal oxide is dissolved in water. Bases include the oxides, hydroxides and carbonates of. Bases often have a bitter taste. Are Bases Soluble In Bases.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog Are Bases Soluble In Bases Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. The determining factor for the result is the solubility. Bases can be classified as soluble and insoluble. However, if a base does dissolve in water, we also call it an. Bases bases have properties that mostly contrast with those of acids. An alkaline solution. Are Bases Soluble In Bases.
From studylib.net
Acids and Bases Are Bases Soluble In Bases These are also called alkalis. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just as acids can. Sodium hydroxide (naoh), potassium hydroxide (koh) Bases often have a bitter taste and are found in foods Most bases are minerals. Are Bases Soluble In Bases.
From crosswordlabs.com
Acids, Bases and Salts Crossword Labs Are Bases Soluble In Bases Sodium hydroxide (naoh), potassium hydroxide (koh) These are also called alkalis. Bases include the oxides, hydroxides and carbonates of. Bases often have a bitter taste and are found in foods The determining factor for the result is the solubility. Bases can be classified as soluble and insoluble. An alkaline solution can be formed when a metal oxide is dissolved in. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID Are Bases Soluble In Bases The determining factor for the result is the solubility. These are also called alkalis. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Most bases are minerals that react with acids to form water and salts. These are bases which are dissolvable in water. Aqueous solutions of bases are also electrolytes. However, if. Are Bases Soluble In Bases.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Are Bases Soluble In Bases Bases can be classified as soluble and insoluble. These are also called alkalis. Sodium hydroxide (naoh), potassium hydroxide (koh) An alkaline solution can be formed when a metal oxide is dissolved in water. When a substance is mixed with a solvent, there are several possible results. Bases include the oxides, hydroxides and carbonates of. Bases can be either strong or. Are Bases Soluble In Bases.
From www.slideserve.com
PPT Acids and Bases Properties, Testing, and pH Scale PowerPoint Are Bases Soluble In Bases The determining factor for the result is the solubility. Bases can be either strong or weak, just as acids can. Bases often have a bitter taste and are found in foods Bases can be classified as soluble and insoluble. An alkaline solution can be formed when a metal oxide is dissolved in water. These are bases which are dissolvable in. Are Bases Soluble In Bases.
From www.youtube.com
Bases and alkalis YouTube Are Bases Soluble In Bases Bases include the oxides, hydroxides and carbonates of. Bases often have a bitter taste and are found in foods These are also called alkalis. These are bases which are dissolvable in water. Bases can be classified as soluble and insoluble. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. An alkaline solution can. Are Bases Soluble In Bases.
From webmis.highland.cc.il.us
Aqueous Solutions Are Bases Soluble In Bases Bases can be either strong or weak, just as acids can. Most bases are minerals that react with acids to form water and salts. Aqueous solutions of bases are also electrolytes. These are bases which are dissolvable in water. Bases bases have properties that mostly contrast with those of acids. Bases include the oxides, hydroxides and carbonates of. Bases can. Are Bases Soluble In Bases.
From www.science-revision.co.uk
alkalis and bases Are Bases Soluble In Bases However, if a base does dissolve in water, we also call it an. Bases include the oxides, hydroxides and carbonates of. These are also called alkalis. When a substance is mixed with a solvent, there are several possible results. Bases can be classified as soluble and insoluble. Most bases are minerals that react with acids to form water and salts.. Are Bases Soluble In Bases.
From askfilo.com
Which is a soluble bases in water? Filo Are Bases Soluble In Bases Bases include the oxides, hydroxides and carbonates of. Sodium hydroxide (naoh), potassium hydroxide (koh) However, if a base does dissolve in water, we also call it an. Bases which are soluble in water form alkalis (soluble bases) some common household products are bases. Bases can be either strong or weak, just as acids can. These are also called alkalis. Most. Are Bases Soluble In Bases.
From www.researchgate.net
(PDF) WaterSoluble Vitamins Bases for Suggested Upper Limits for Are Bases Soluble In Bases The determining factor for the result is the solubility. When a substance is mixed with a solvent, there are several possible results. However, if a base does dissolve in water, we also call it an. These are bases which are dissolvable in water. Bases include the oxides, hydroxides and carbonates of. Sodium hydroxide (naoh), potassium hydroxide (koh) An alkaline solution. Are Bases Soluble In Bases.
From stock.adobe.com
Stockvector Acids Bases and Salts infographic diagram showing solution Are Bases Soluble In Bases Bases bases have properties that mostly contrast with those of acids. Bases can be either strong or weak, just as acids can. These are bases which are dissolvable in water. Bases can be classified as soluble and insoluble. An alkaline solution can be formed when a metal oxide is dissolved in water. When a substance is mixed with a solvent,. Are Bases Soluble In Bases.