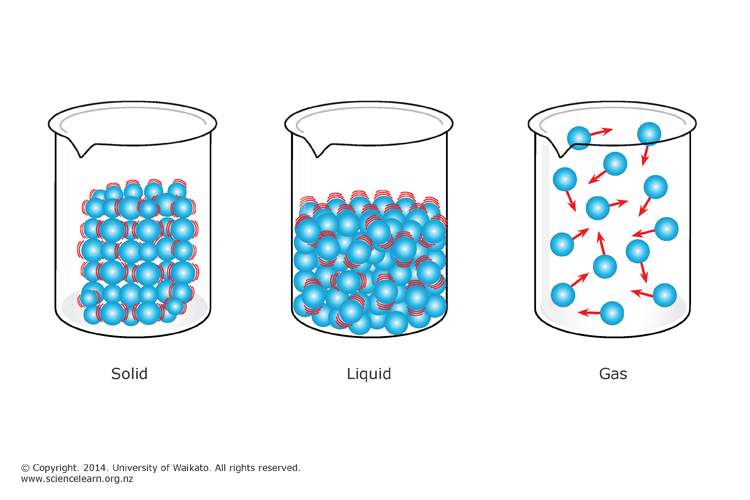
Science Definition Kinetic Theory . The kinetic particle model explains the properties of the different states of matter. The motion of molecules in a gas is random in magnitude. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To use kinetic molecular theory to describe the behavior of the macroscopic. To understand the five fundamentals of kinetic molecular theory. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. Particles in solids, liquids and gases have different amounts of energy. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that.
from www.sciencelearn.org.nz
The kinetic particle model explains the properties of the different states of matter. To use kinetic molecular theory to describe the behavior of the macroscopic. To understand the five fundamentals of kinetic molecular theory. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The motion of molecules in a gas is random in magnitude. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. Particles in solids, liquids and gases have different amounts of energy. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that.
model of matter — Science Learning Hub
Science Definition Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The motion of molecules in a gas is random in magnitude. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To understand the five fundamentals of kinetic molecular theory. Particles in solids, liquids and gases have different amounts of energy. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. To use kinetic molecular theory to describe the behavior of the macroscopic. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. The kinetic particle model explains the properties of the different states of matter.
From mmerevise.co.uk
Molecular Theory Model Worksheets, Questions and Revision MME Science Definition Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The motion of molecules in a gas is random in magnitude. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. A theory that the particles of a gas move in straight lines with. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Theory and Gases PowerPoint Presentation, free download Science Definition Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. To understand the five fundamentals of kinetic molecular theory. The kinetic particle model explains the properties of the different states of matter. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory states that. Science Definition Kinetic Theory.
From www.madebyteachers.com
Let's Talk Vocab...Chemistry Theory of Matter Worksheet Made Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. To understand the five fundamentals of kinetic molecular theory. Particles in solids, liquids and gases have different. Science Definition Kinetic Theory.
From www.sciencelearn.org.nz
model of matter — Science Learning Hub Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. Particles in solids, liquids and gases have different amounts of energy. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic theory states that. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Theory The Microscopic Macroscopic Connection PowerPoint Science Definition Kinetic Theory To use kinetic molecular theory to describe the behavior of the macroscopic. To understand the five fundamentals of kinetic molecular theory. Particles in solids, liquids and gases have different amounts of energy. The kinetic particle model explains the properties of the different states of matter. A theory that the particles of a gas move in straight lines with high average. Science Definition Kinetic Theory.
From ar.inspiredpencil.com
Definition Of Energy Science Definition Kinetic Theory To understand the five fundamentals of kinetic molecular theory. To use kinetic molecular theory to describe the behavior of the macroscopic. The motion of molecules in a gas is random in magnitude. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. A theory that the particles of a. Science Definition Kinetic Theory.
From www.sciencefacts.net
Energy Definition, Formula, Examples, & Pictures Science Definition Kinetic Theory The kinetic particle model explains the properties of the different states of matter. Particles in solids, liquids and gases have different amounts of energy. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory relates the independent motion of molecules to the mechanical and thermal. Science Definition Kinetic Theory.
From slideplayer.com
III. States of Matter Molecular Theory States of Matter ppt Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. The motion of molecules in a gas is random in magnitude. Kinetic theory. Science Definition Kinetic Theory.
From www.physiquechimiemathbiologie.com
Course Theory And Statistical Mechanics PDF Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To use kinetic molecular theory to describe the behavior of the macroscopic. To understand the five fundamentals of kinetic molecular theory. The kinetic theory states that the temperature of a substance is a measure of the average kinetic. Science Definition Kinetic Theory.
From www.slideshare.net
Theory Of Matter Science Definition Kinetic Theory Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The motion of molecules in a gas is random in magnitude. Particles in solids, liquids and gases have different amounts of energy. The kinetic theory states that the temperature of a substance is a measure of the average kinetic. Science Definition Kinetic Theory.
From www.grc.nasa.gov
Theory of Gases Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic particle model explains the properties of the different states of matter. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The motion of. Science Definition Kinetic Theory.
From slidetodoc.com
Chapter 10 The Theory of Matter Section Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic theory states that the temperature of a substance is a measure of the average. Science Definition Kinetic Theory.
From www.slideserve.com
PPT The Theory of Matter explains the properties of solids Science Definition Kinetic Theory The kinetic particle model explains the properties of the different states of matter. The motion of molecules in a gas is random in magnitude. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. The kinetic theory relates the independent motion of molecules to the. Science Definition Kinetic Theory.
From gbu-taganskij.ru
PPT Molecular Theory (KMT) PowerPoint Presentation,, 40 OFF Science Definition Kinetic Theory To use kinetic molecular theory to describe the behavior of the macroscopic. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The motion of molecules in a gas is random in magnitude. The kinetic particle model explains the properties of the different states of matter. To understand. Science Definition Kinetic Theory.
From www.vrogue.co
Energy Definition Formula Examples Teachoo vrogue.co Science Definition Kinetic Theory The kinetic particle model explains the properties of the different states of matter. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The motion of molecules in a gas is random in magnitude. The kinetic theory states that the temperature of a substance is a measure of. Science Definition Kinetic Theory.
From sciencenotes.org
Molecular Theory of Gases Science Definition Kinetic Theory To understand the five fundamentals of kinetic molecular theory. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The motion of molecules in a gas is. Science Definition Kinetic Theory.
From www.achieversdream.com.sg
About Particle Theory Achievers Dream Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. Particles in solids, liquids and gases have different amounts of energy. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. Kinetic theory. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Theory and Gases PowerPoint Presentation, free download Science Definition Kinetic Theory Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. To understand the five fundamentals of kinetic molecular theory. The motion of molecules in a gas is. Science Definition Kinetic Theory.
From sciencenotes.org
What Is Energy? Energy Examples Science Definition Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The kinetic particle model explains the properties of the different states of matter. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To understand the five fundamentals of kinetic molecular theory. The motion of molecules in. Science Definition Kinetic Theory.
From www.careerpower.in
Energy Definition, Example and Derivation Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic particle model explains the properties of the different states of matter. The motion of molecules in a gas is random in magnitude. Particles in solids, liquids and gases have different amounts of energy. A theory that. Science Definition Kinetic Theory.
From gamesmartz.com
Theory Definition & Image GameSmartz Science Definition Kinetic Theory The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. To use kinetic molecular theory to describe the behavior of the macroscopic. To understand the five fundamentals of kinetic molecular theory. The kinetic theory relates the independent motion of molecules to the mechanical and thermal. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic particle model explains the properties of the different states of matter. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The motion of molecules. Science Definition Kinetic Theory.
From www.mdpi.com
TheoryBased Methods in Fluid Dynamics MDPI Books Science Definition Kinetic Theory To understand the five fundamentals of kinetic molecular theory. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. Particles in solids, liquids and gases have different amounts of energy. The kinetic particle model explains the properties of the different states of matter. The kinetic theory relates the independent. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Molecular Theory PowerPoint Presentation, free download Science Definition Kinetic Theory The motion of molecules in a gas is random in magnitude. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic particle model explains the properties of the different states of matter. Kinetic theory of gases, a theory based on a simplified molecular or particle description. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Theory The Microscopic Macroscopic Connection PowerPoint Science Definition Kinetic Theory The kinetic particle model explains the properties of the different states of matter. To use kinetic molecular theory to describe the behavior of the macroscopic. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The kinetic theory relates the independent motion of molecules to the mechanical and thermal. Science Definition Kinetic Theory.
From www.sliderbase.com
Molecular Theory Presentation Chemistry Science Definition Kinetic Theory The motion of molecules in a gas is random in magnitude. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. To use kinetic molecular theory to describe the behavior of the macroscopic. Kinetic theory of gases, a theory based on a simplified molecular or. Science Definition Kinetic Theory.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Science Definition Kinetic Theory Particles in solids, liquids and gases have different amounts of energy. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To use kinetic molecular theory to describe the behavior of the macroscopic. The kinetic particle model explains the properties of the different states of matter. The motion. Science Definition Kinetic Theory.
From askfilo.com
Examples based on Theory of Cases 8 Interpretation of Tem.. Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. To use kinetic molecular theory to describe the behavior of the macroscopic. The kinetic particle model explains the properties of the different states of matter. Particles in solids, liquids and gases have different amounts of energy. A theory. Science Definition Kinetic Theory.
From gamesmartz.com
Energy Definition & Image GameSmartz Science Definition Kinetic Theory The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. Kinetic theory of gases, a theory based on a simplified molecular or particle. Science Definition Kinetic Theory.
From www.slideserve.com
PPT The Theory of Matter PowerPoint Presentation Science Definition Kinetic Theory To understand the five fundamentals of kinetic molecular theory. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The motion of molecules in. Science Definition Kinetic Theory.
From gideonkruwcantrell.blogspot.com
Describe Solids Liquids and Gases Using the Theory Science Definition Kinetic Theory Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. Particles in solids, liquids and gases have different amounts of energy. To understand the five fundamentals of kinetic molecular theory. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of. Science Definition Kinetic Theory.
From www.slideshare.net
Molecular Theory Science Definition Kinetic Theory To understand the five fundamentals of kinetic molecular theory. A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. To use kinetic molecular theory to describe the behavior of the macroscopic. The kinetic particle model explains the properties of the different states of matter. Kinetic theory of gases,. Science Definition Kinetic Theory.
From www.simply.science
Respiratory System Science Definition Kinetic Theory Kinetic theory of gases, a theory based on a simplified molecular or particle description of a gas, from which many gross. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. Particles in solids, liquids and gases have different amounts of energy. The motion of molecules in a. Science Definition Kinetic Theory.
From cassiusyouthmorrison.blogspot.com
Which Is the Best Summary of the Theory Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory states that the temperature of a substance is a measure of the average kinetic energy of the particles that make up that. Particles in solids, liquids and gases have different amounts of energy. The kinetic. Science Definition Kinetic Theory.
From ar.inspiredpencil.com
Molecular Theory Examples Science Definition Kinetic Theory A theory that the particles of a gas move in straight lines with high average velocity, continually encounter one another and thus. The kinetic theory relates the independent motion of molecules to the mechanical and thermal properties of gases—namely, their pressure, volume, temperature, viscosity,. Particles in solids, liquids and gases have different amounts of energy. The motion of molecules in. Science Definition Kinetic Theory.