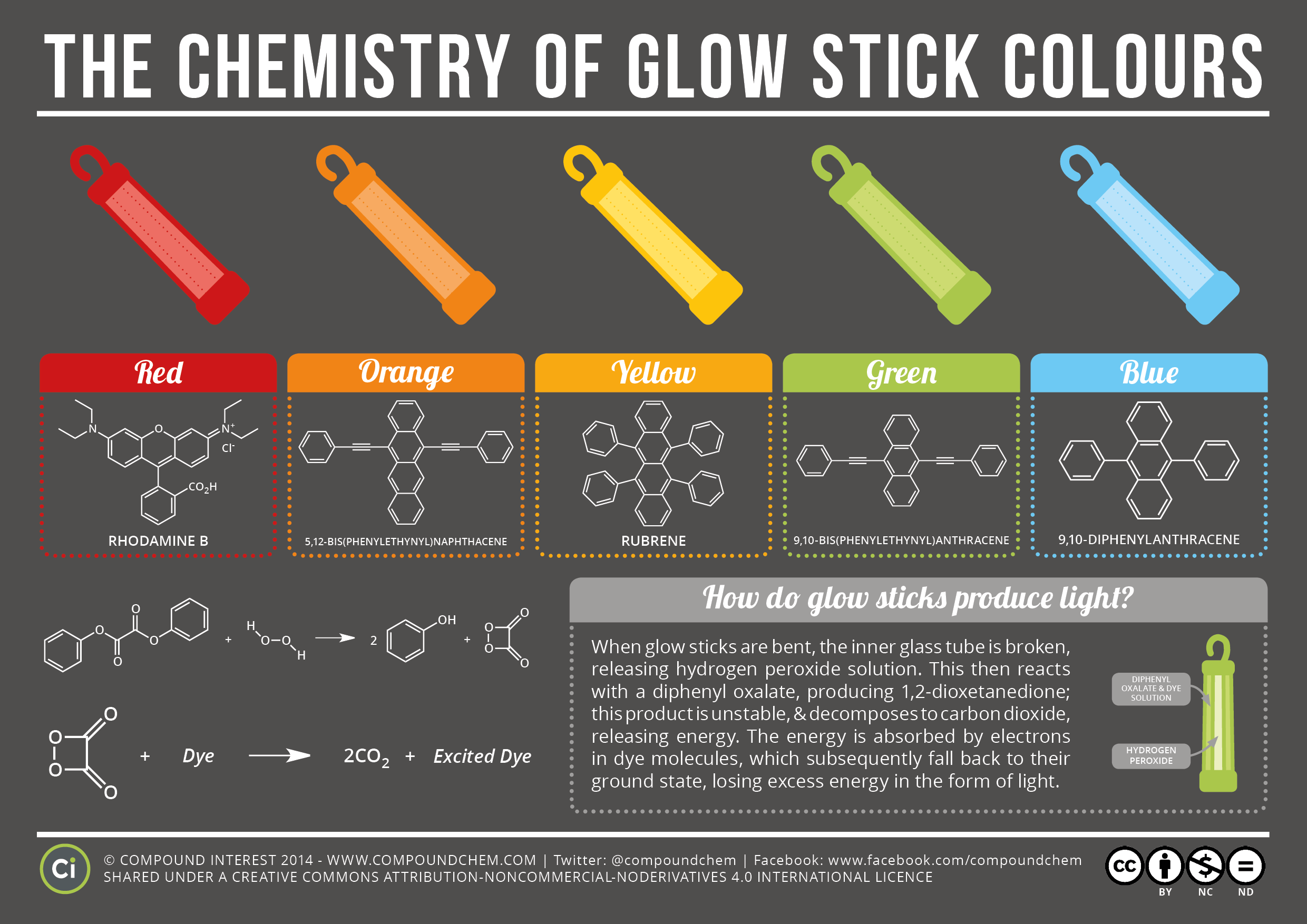
What Chemical Are In Glow Stick . One solution, in the case of most glow sticks, contains a diphenyl oxalate. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. A glow stick is a light source based on chemiluminescence. That light is made by a chemical reaction—a. The cyclic peroxy compound decomposes to carbon dioxide. Glow sticks actually contain two separate compartments, with two different chemical solutions. Specifically, the chemical reaction works like this: The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. The resulting chemical reaction causes a fluorescent dye to emit light. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or are unsuitable for. Glow sticks are not poisonous.
from www.reddit.com
The resulting chemical reaction causes a fluorescent dye to emit light. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. Glow sticks actually contain two separate compartments, with two different chemical solutions. A glow stick is a light source based on chemiluminescence. Glow sticks are not poisonous. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. That light is made by a chemical reaction—a. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in the case of most glow sticks, contains a diphenyl oxalate. Specifically, the chemical reaction works like this:
chemistry of glow stick colors (infographic) EverythingScience
What Chemical Are In Glow Stick Glow sticks are not poisonous. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. That light is made by a chemical reaction—a. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. Glow sticks are not poisonous. The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. One solution, in the case of most glow sticks, contains a diphenyl oxalate. The resulting chemical reaction causes a fluorescent dye to emit light. Specifically, the chemical reaction works like this: The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or are unsuitable for. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick is a light source based on chemiluminescence. Glow sticks actually contain two separate compartments, with two different chemical solutions.
From www.reddit.com
chemistry of glow stick colors (infographic) EverythingScience What Chemical Are In Glow Stick Glow sticks actually contain two separate compartments, with two different chemical solutions. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. Snapping the stick breaks an inner container filled with hydrogen peroxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. The. What Chemical Are In Glow Stick.
From www.pinterest.com
Glow Stick Reactions Science Experiment Science demonstrations What Chemical Are In Glow Stick The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The cyclic peroxy compound decomposes to carbon dioxide. Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. That light. What Chemical Are In Glow Stick.
From www.compoundchem.com
The Chemistry of Glow Sticks Compound Interest What Chemical Are In Glow Stick A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. The resulting chemical reaction causes a fluorescent dye to emit light. The cyclic peroxy compound decomposes to carbon dioxide. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. The unstable. What Chemical Are In Glow Stick.
From www.pinterest.com
Chemical Reactions Glow Stick Science Investigate Light With Grade 2 What Chemical Are In Glow Stick Specifically, the chemical reaction works like this: Snapping the stick breaks an inner container filled with hydrogen peroxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. Glow sticks actually contain two separate compartments, with two different chemical solutions. A glow stick is a light source based on chemiluminescence. One solution, in the. What Chemical Are In Glow Stick.
From www.adabofgluewilldo.com
Glow Stick Science Chemical Reaction Lab What Chemical Are In Glow Stick Glow sticks actually contain two separate compartments, with two different chemical solutions. Glow sticks are not poisonous. One solution, in the case of most glow sticks, contains a diphenyl oxalate. The resulting chemical reaction causes a fluorescent dye to emit light. Snapping the stick breaks an inner container filled with hydrogen peroxide. Specifically, the chemical reaction works like this: When. What Chemical Are In Glow Stick.
From www.instructables.com
Make Glow Sticks the Science (with Pictures) What Chemical Are In Glow Stick Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or. What Chemical Are In Glow Stick.
From www.ingridscience.ca
Glow stick chemistry ingridscience.ca What Chemical Are In Glow Stick One solution, in the case of most glow sticks, contains a diphenyl oxalate. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl. What Chemical Are In Glow Stick.
From www.slideserve.com
PPT Chemical Reactions Glow Sticks PowerPoint Presentation, free What Chemical Are In Glow Stick Glow sticks actually contain two separate compartments, with two different chemical solutions. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in. What Chemical Are In Glow Stick.
From www.lihpao.com
How Does a Glow Stick Work? Exploring the Chemistry and Physics Behind What Chemical Are In Glow Stick That light is made by a chemical reaction—a. Specifically, the chemical reaction works like this: The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. A glow stick consists of a glass vial,. What Chemical Are In Glow Stick.
From byjus.com
GlowInTheDark Chemistry Explained! Learning Tree What Chemical Are In Glow Stick Glow sticks are not poisonous. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. A glow stick is a light source based on chemiluminescence. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. That light is made by a. What Chemical Are In Glow Stick.
From teachingscience.us
FREE Science Experiment with Glow Sticks What Chemical Are In Glow Stick When you bend the plastic vial, the glass vial breaks and the two solutions flow together. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or are unsuitable for.. What Chemical Are In Glow Stick.
From www.youtube.com
Chemical reaction of glow stick including rate of reaction YouTube What Chemical Are In Glow Stick The cyclic peroxy compound decomposes to carbon dioxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. A glow stick is a light source based on chemiluminescence. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. The unstable peroxyacid. What Chemical Are In Glow Stick.
From www.adabofgluewilldo.com
Glow Stick Science Chemical Reaction Lab A Dab of Glue Will Do What Chemical Are In Glow Stick The cyclic peroxy compound decomposes to carbon dioxide. That light is made by a chemical reaction—a. A glow stick is a light source based on chemiluminescence. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. However, glow. What Chemical Are In Glow Stick.
From facts.net
101 Fun Chemistry Facts Your Teachers Don't Tell You What Chemical Are In Glow Stick Glow sticks actually contain two separate compartments, with two different chemical solutions. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The cyclic peroxy compound decomposes to carbon dioxide. Glow sticks are not poisonous. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Specifically, the chemical reaction works like. What Chemical Are In Glow Stick.
From shop.scholastic.com
Glow Stick Science Experiment for Kids What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Specifically, the chemical reaction works like this: Glow sticks actually contain two separate compartments, with two different chemical solutions. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found. What Chemical Are In Glow Stick.
From astrocamp.org
How Do Glow Sticks Work? AstroCamp Science Camp What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. That light is made by a chemical reaction—a. The hydrogen peroxide oxidizes the phenyl oxalate ester,. What Chemical Are In Glow Stick.
From www.ingridscience.ca
Glow stick chemistry ingridscience.ca What Chemical Are In Glow Stick The cyclic peroxy compound decomposes to carbon dioxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. A glow stick is a light source based on chemiluminescence. Snapping the stick breaks an. What Chemical Are In Glow Stick.
From express.adobe.com
Chemical Reactions in Glow sticks What Chemical Are In Glow Stick The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Snapping the stick breaks an inner container filled with hydrogen peroxide. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. A glow stick is a light source based on chemiluminescence. Glow sticks are not poisonous. However, glow stick reactions often. What Chemical Are In Glow Stick.
From www.ingridscience.ca
Glow stick chemistry ingridscience.ca What Chemical Are In Glow Stick Glow sticks are not poisonous. One solution, in the case of most glow sticks, contains a diphenyl oxalate. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. However, glow stick. What Chemical Are In Glow Stick.
From www.youtube.com
DIY Glow Stick (Chemical way) YouTube What Chemical Are In Glow Stick The resulting chemical reaction causes a fluorescent dye to emit light. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. The cyclic peroxy compound decomposes to carbon dioxide. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Specifically, the chemical reaction works. What Chemical Are In Glow Stick.
From chemceed.com
The Science of Chem Light ChemCeed What Chemical Are In Glow Stick When you bend the plastic vial, the glass vial breaks and the two solutions flow together. That light is made by a chemical reaction—a. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. A glow stick is a light source based on chemiluminescence. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an. What Chemical Are In Glow Stick.
From blogs.ubc.ca
Glow sticks and luminescence The Undergraduate Scientist What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Snapping the stick breaks an inner container filled with hydrogen peroxide. One solution, in the case of most glow sticks, contains a diphenyl oxalate. When you bend the plastic vial, the glass vial breaks and the two solutions flow. What Chemical Are In Glow Stick.
From www.chemistry-blog.com
ChemSummer Carnival Glow Sticks How Do They Work? Chemistry Blog What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Glow sticks are not poisonous. The resulting chemical reaction causes a fluorescent dye to emit light. Snapping the stick breaks an inner container filled with hydrogen peroxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and. What Chemical Are In Glow Stick.
From www.fishingtackleshop.com.au
Chemical Light Stick Night Glow Stick (Pack of 6) What Chemical Are In Glow Stick However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or are unsuitable for. Snapping the stick breaks an inner container filled with hydrogen peroxide. The cyclic peroxy compound decomposes to carbon dioxide. The resulting chemical reaction causes a fluorescent dye to emit. What Chemical Are In Glow Stick.
From www.ingridscience.ca
Glow stick chemistry ingridscience.ca What Chemical Are In Glow Stick Glow sticks are not poisonous. Specifically, the chemical reaction works like this: The resulting chemical reaction causes a fluorescent dye to emit light. That light is made by a chemical reaction—a. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Snapping the stick breaks an inner container filled. What Chemical Are In Glow Stick.
From www.youtube.com
Make Reusable Glow Sticks Safe Easy & No Chemicals Long Lasting What Chemical Are In Glow Stick Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick is a light source based on chemiluminescence. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. One solution, in. What Chemical Are In Glow Stick.
From krmajgwezp.blogspot.com
How Do Glow Sticks Work Chemistry Hydrogen peroxide and a phenyl What Chemical Are In Glow Stick The resulting chemical reaction causes a fluorescent dye to emit light. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. A glow stick is a light source based on chemiluminescence. The unstable peroxyacid ester decomposes, resulting in phenol and a cyclic peroxy compound. Snapping the stick breaks an inner container filled with hydrogen. What Chemical Are In Glow Stick.
From cen.acs.org
What are glow sticks, and what’s the chemical reaction that makes them What Chemical Are In Glow Stick A glow stick is a light source based on chemiluminescence. However, glow stick reactions often involve expensive dyes and the use of solvents that are either unlikely to be found in a typical school store (diethyl phthalate), or are unsuitable for. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting. What Chemical Are In Glow Stick.
From www.slideserve.com
PPT Chemical Reactions Glow Sticks PowerPoint Presentation ID1171343 What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Snapping the stick breaks an inner container filled with hydrogen peroxide. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. Glow sticks actually contain two separate compartments, with two different chemical solutions.. What Chemical Are In Glow Stick.
From www.adabofgluewilldo.com
Glow Stick Science Chemical Reaction Lab What Chemical Are In Glow Stick Snapping the stick breaks an inner container filled with hydrogen peroxide. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. Glow sticks are not poisonous. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. That light is made by. What Chemical Are In Glow Stick.
From www.thefactsite.com
Brighten Up Your Day With Glow Stick Facts The Fact Site What Chemical Are In Glow Stick The cyclic peroxy compound decomposes to carbon dioxide. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube is bent, resulting in a luminescent glow. When you bend the plastic vial, the glass vial breaks and the two solutions flow together. Glow sticks actually contain two separate compartments, with two different chemical solutions. Specifically,. What Chemical Are In Glow Stick.
From www.youtube.com
Make a glow stick reaction with real chemicals YouTube What Chemical Are In Glow Stick Snapping the stick breaks an inner container filled with hydrogen peroxide. A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. Glow sticks are not poisonous. One solution, in the case of most glow sticks, contains a diphenyl oxalate. The cyclic peroxy compound decomposes to carbon dioxide. A glow. What Chemical Are In Glow Stick.
From studylib.net
Chemical Reactions Glow Sticks What Chemical Are In Glow Stick Snapping the stick breaks an inner container filled with hydrogen peroxide. The resulting chemical reaction causes a fluorescent dye to emit light. The cyclic peroxy compound decomposes to carbon dioxide. That light is made by a chemical reaction—a. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. One solution, in the case of. What Chemical Are In Glow Stick.
From www.pinterest.com
Chemical Reactions Glow Stick Science Investigate Light Grade 2 3 What Chemical Are In Glow Stick Glow sticks actually contain two separate compartments, with two different chemical solutions. One solution, in the case of most glow sticks, contains a diphenyl oxalate. That light is made by a chemical reaction—a. Glow sticks are not poisonous. The hydrogen peroxide oxidizes the phenyl oxalate ester, to form phenol and an unstable peroxyacid ester. The resulting chemical reaction causes a. What Chemical Are In Glow Stick.
From express.adobe.com
Glow Sticks Chemical Reaction What Chemical Are In Glow Stick A glow stick consists of a glass vial, containing one chemical solution, housed inside a larger plastic vial, containing another solution. The resulting chemical reaction causes a fluorescent dye to emit light. Glow sticks actually contain two separate compartments, with two different chemical solutions. A glow stick is a plastic tube containing two reactive chemicals, which combine when the tube. What Chemical Are In Glow Stick.