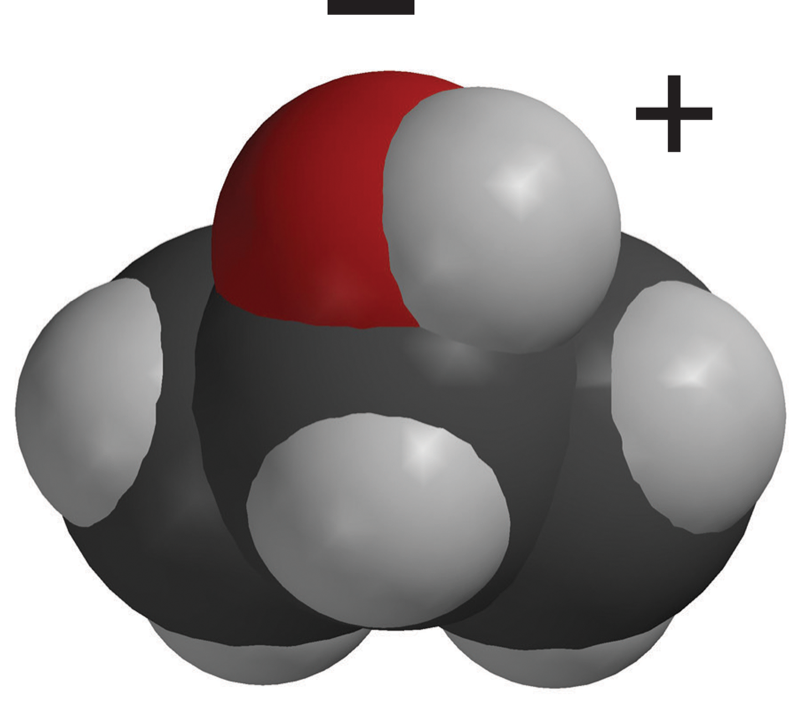
Do All Alcohols Dissolve In Water . Mixing the two in any proportion generates a single solution. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Alcohols can be considered derivatives of. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. See the solubility chart and examples of primary, secondary and tertiary alcohols. Whatever proportions you mix them in, you will get a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Methanol, ethanol and propanol are completely. Solubility of alcohols in water. Find out how temperature, ph, and. However, solubility decreases as the length of the hydrocarbon chain in the. The small alcohols are completely soluble in water. Small alcohols are completely soluble in water;
from www.acs.org
Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Solubility of alcohols in water. Alcohols can be considered derivatives of. The small alcohols are completely soluble in water. See the solubility chart and examples of primary, secondary and tertiary alcohols. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. However, solubility decreases as the length of the hydrocarbon chain in the. Find out how temperature, ph, and. Methanol, ethanol and propanol are completely.
Lesson 5.4 Why Does Water Dissolve Sugar? American Chemical Society
Do All Alcohols Dissolve In Water Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Whatever proportions you mix them in, you will get a single solution. Small alcohols are completely soluble in water; Mixing the two in any proportion generates a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. See the solubility chart and examples of primary, secondary and tertiary alcohols. Alcohols can be considered derivatives of. Find out how temperature, ph, and. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. However, solubility decreases as the length of the hydrocarbon chain in the. Methanol, ethanol and propanol are completely. The small alcohols are completely soluble in water. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Solubility of alcohols in water.
From www.acs.org
Lesson 5.4 Why Does Water Dissolve Sugar? American Chemical Society Do All Alcohols Dissolve In Water Whatever proportions you mix them in, you will get a single solution. Alcohols can be considered derivatives of. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. See the solubility chart and examples of primary, secondary and tertiary alcohols. Solubility of alcohols in water. Find out how temperature, ph, and. Explain why alcohols. Do All Alcohols Dissolve In Water.
From www.slideshare.net
Alcohol powerpoint Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The small alcohols are completely soluble in water. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how. Do All Alcohols Dissolve In Water.
From www.chegg.com
Solved Which alcohol is most soluble in water? Multiple Do All Alcohols Dissolve In Water Mixing the two in any proportion generates a single solution. Find out how temperature, ph, and. Methanol, ethanol and propanol are completely. See the solubility chart and examples of primary, secondary and tertiary alcohols. Small alcohols are completely soluble in water; Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on. Do All Alcohols Dissolve In Water.
From slidetodoc.com
Chapter 13 Alcohols Phenols and Thiols 13 3 Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. The small alcohols are completely soluble in water. Methanol, ethanol and propanol are completely. Alcohols can be considered derivatives of. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Learn how bond dipoles,. Do All Alcohols Dissolve In Water.
From www.youtube.com
Polyvinyl alcohol PVA dissolving in water timelapse YouTube Do All Alcohols Dissolve In Water Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. See the solubility chart and examples of primary, secondary and tertiary alcohols. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable. Do All Alcohols Dissolve In Water.
From www.youtube.com
4 2 4 alcohols solubility and solvent polarity YouTube Do All Alcohols Dissolve In Water The small alcohols are completely soluble in water. Methanol, ethanol and propanol are completely. Find out how temperature, ph, and. Mixing the two in any proportion generates a single solution. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Alcohol PowerPoint Presentation, free download ID234072 Do All Alcohols Dissolve In Water Find out how temperature, ph, and. However, solubility decreases as the length of the hydrocarbon chain in the. Whatever proportions you mix them in, you will get a single solution. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Methanol, ethanol and propanol are. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Alcohol reactions PowerPoint Presentation, free download ID3914237 Do All Alcohols Dissolve In Water Alcohols can be considered derivatives of. The small alcohols are completely soluble in water. Mixing the two in any proportion generates a single solution. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Explain why alcohols. Do All Alcohols Dissolve In Water.
From builderbold.com
Does salt dissolve in alcohol? BuilderBold Do All Alcohols Dissolve In Water Alcohols can be considered derivatives of. See the solubility chart and examples of primary, secondary and tertiary alcohols. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Small alcohols are completely soluble in water; Whatever proportions. Do All Alcohols Dissolve In Water.
From slideplayer.com
Organic Chemistry Topic 11 Text Chapters ppt download Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Solubility of alcohols in water. Small alcohols are completely soluble in water; Whatever proportions you mix them in, you will get a single solution. See the solubility chart and examples of primary, secondary and tertiary alcohols. Learn how the. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 Do All Alcohols Dissolve In Water Small alcohols are completely soluble in water; Methanol, ethanol and propanol are completely. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Solubility of alcohols in water. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Explain why alcohols and ethers of four or fewer carbon atoms. Do All Alcohols Dissolve In Water.
From 2012books.lardbucket.org
Physical Properties of Alcohols Do All Alcohols Dissolve In Water Alcohols can be considered derivatives of. Methanol, ethanol and propanol are completely. However, solubility decreases as the length of the hydrocarbon chain in the. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on. Do All Alcohols Dissolve In Water.
From www.transtutors.com
(Solved) Which Alcohol Is Most Soluble In Water? Multiple Choice... (1 Answer) Transtutors Do All Alcohols Dissolve In Water Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Find out how temperature, ph, and. See the solubility chart and examples of primary, secondary and tertiary alcohols. Alcohols can be considered derivatives of. However, solubility decreases as the length of the hydrocarbon chain in the. Whatever proportions you mix them in, you will get. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Principles of Solubility Nature of the solute and solvent PowerPoint Presentation ID6444367 Do All Alcohols Dissolve In Water Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Small alcohols are completely soluble in water; Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Learn how the solubility of alcohols in water depends on their carbon chain. Do All Alcohols Dissolve In Water.
From www.thesciencehive.co.uk
Alcohols* — the science sauce Do All Alcohols Dissolve In Water Small alcohols are completely soluble in water; Learn how the size, structure, and functional groups of alcohols affect their solubility in water. See the solubility chart and examples of primary, secondary and tertiary alcohols. The small alcohols are completely soluble in water. Solubility of alcohols in water. However, solubility decreases as the length of the hydrocarbon chain in the. Methanol,. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6540865 Do All Alcohols Dissolve In Water Learn how the size, structure, and functional groups of alcohols affect their solubility in water. However, solubility decreases as the length of the hydrocarbon chain in the. Alcohols can be considered derivatives of. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Whatever proportions you mix them in,. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Factors That Affect Solubility and Rate of Dissolving PowerPoint Presentation ID4066317 Do All Alcohols Dissolve In Water Solubility of alcohols in water. Find out how temperature, ph, and. Alcohols can be considered derivatives of. The small alcohols are completely soluble in water. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Small alcohols are completely soluble in water; Whatever proportions you mix them in, you will get a single solution.. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2736092 Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Small alcohols are completely soluble in water; Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Solubility of alcohols in water. However, solubility decreases as the length of the hydrocarbon chain. Do All Alcohols Dissolve In Water.
From yesdirt.com
Does Alcohol Dissolve in Water? (ANSWERED) Yes Dirt Do All Alcohols Dissolve In Water Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes. Do All Alcohols Dissolve In Water.
From www.chemistrystudent.com
Alcohols (ALevel) ChemistryStudent Do All Alcohols Dissolve In Water Mixing the two in any proportion generates a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Methanol, ethanol and propanol are completely. See the solubility chart and examples of primary, secondary and tertiary alcohols. Whatever proportions you mix them in, you will get a single. Do All Alcohols Dissolve In Water.
From fyoafudxu.blob.core.windows.net
Does Alcohol Soluble In Water at Walter Fox blog Do All Alcohols Dissolve In Water Alcohols can be considered derivatives of. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Small alcohols are completely soluble in water; Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. The small alcohols are completely soluble in water. Mixing the two in any proportion. Do All Alcohols Dissolve In Water.
From byjus.com
Among primary, secondary and tertiary, which alcohol is more soluble in water?? Do All Alcohols Dissolve In Water Whatever proportions you mix them in, you will get a single solution. See the solubility chart and examples of primary, secondary and tertiary alcohols. Methanol, ethanol and propanol are completely. Solubility of alcohols in water. Mixing the two in any proportion generates a single solution. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds. Do All Alcohols Dissolve In Water.
From www.masterorganicchemistry.com
Elimination Reactions of Alcohols — Master Organic Chemistry Do All Alcohols Dissolve In Water Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Alcohols can be considered derivatives of. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Mixing the two in any proportion generates a single solution. Learn how alcohols are soluble in water due to hydrogen bonding and polarity,. Do All Alcohols Dissolve In Water.
From slideplayer.com
Chapter Nine Solutions Fundamentals of General, Organic, and Biological Chemistry 7th Edition Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. However, solubility decreases as the length of the hydrocarbon chain in the. Mixing the two in any proportion generates a single solution. Methanol, ethanol and propanol are completely. See the solubility chart and examples of primary, secondary and tertiary. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT ALCOHOL PowerPoint Presentation, free download ID4296802 Do All Alcohols Dissolve In Water However, solubility decreases as the length of the hydrocarbon chain in the. Solubility of alcohols in water. Methanol, ethanol and propanol are completely. Alcohols can be considered derivatives of. Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. Whatever proportions you mix them in, you will get a single solution. The small alcohols. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Solutions and their Behavior PowerPoint Presentation, free download ID1415921 Do All Alcohols Dissolve In Water The small alcohols are completely soluble in water. Whatever proportions you mix them in, you will get a single solution. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Solubility of alcohols in. Do All Alcohols Dissolve In Water.
From www.youtube.com
Physical Properties of Alcohol Hydrogen Bonding, Solubility and Boiling Point YouTube Do All Alcohols Dissolve In Water Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Small alcohols are completely soluble in water; Whatever proportions you mix them in, you will get a single solution. However, solubility decreases as the length of the hydrocarbon chain in the. The small alcohols are completely soluble in water. Solubility of alcohols in water. Learn. Do All Alcohols Dissolve In Water.
From slideplayer.com
melting & boiling points ppt download Do All Alcohols Dissolve In Water Find out how temperature, ph, and. Mixing the two in any proportion generates a single solution. Methanol, ethanol and propanol are completely. The small alcohols are completely soluble in water. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Small alcohols are completely soluble in water; Alcohols can be considered derivatives of. However, solubility. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT 2 Intermolecular forces PowerPoint Presentation, free download ID2740353 Do All Alcohols Dissolve In Water Find out how temperature, ph, and. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Whatever proportions you mix them in, you will get a single solution. The small alcohols are completely soluble in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Physical and Chemical Properties Of Alcohols! PowerPoint Presentation ID3105336 Do All Alcohols Dissolve In Water Methanol, ethanol and propanol are completely. Solubility of alcohols in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. See the solubility chart and examples of primary, secondary and tertiary alcohols. Learn. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Part I Solubility, Factors Affecting Solubility PowerPoint Presentation ID2948662 Do All Alcohols Dissolve In Water Learn how the solubility of alcohols in water depends on their carbon chain length and polarity. The small alcohols are completely soluble in water. Learn how the size, structure, and functional groups of alcohols affect their solubility in water. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon. Do All Alcohols Dissolve In Water.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross out what does not apply Do All Alcohols Dissolve In Water However, solubility decreases as the length of the hydrocarbon chain in the. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Learn how the solubility of alcohols in water depends on. Do All Alcohols Dissolve In Water.
From www.quanswer.com
Why alcohol dissolve in water? Quanswer Do All Alcohols Dissolve In Water However, solubility decreases as the length of the hydrocarbon chain in the. Solubility of alcohols in water. Learn how bond dipoles, hydrogen bonds, and hydrophobic effects determine the solubility of organic compounds in water. Whatever proportions you mix them in, you will get a single solution. Explain why alcohols and ethers of four or fewer carbon atoms are soluble in. Do All Alcohols Dissolve In Water.
From www.slideshare.net
Introduction of alcohol Do All Alcohols Dissolve In Water Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble. Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. Alcohols can be considered derivatives of. Learn how the size, structure, and functional groups. Do All Alcohols Dissolve In Water.
From www.slideserve.com
PPT Factors That Affect Solubility and Rate of Dissolving PowerPoint Presentation ID4066317 Do All Alcohols Dissolve In Water Find out how temperature, ph, and. Small alcohols are completely soluble in water; Learn how alcohols are soluble in water due to hydrogen bonding and polarity, and how the solubility depends on the carbon chain length and branching. The small alcohols are completely soluble in water. Mixing the two in any proportion generates a single solution. However, solubility decreases as. Do All Alcohols Dissolve In Water.