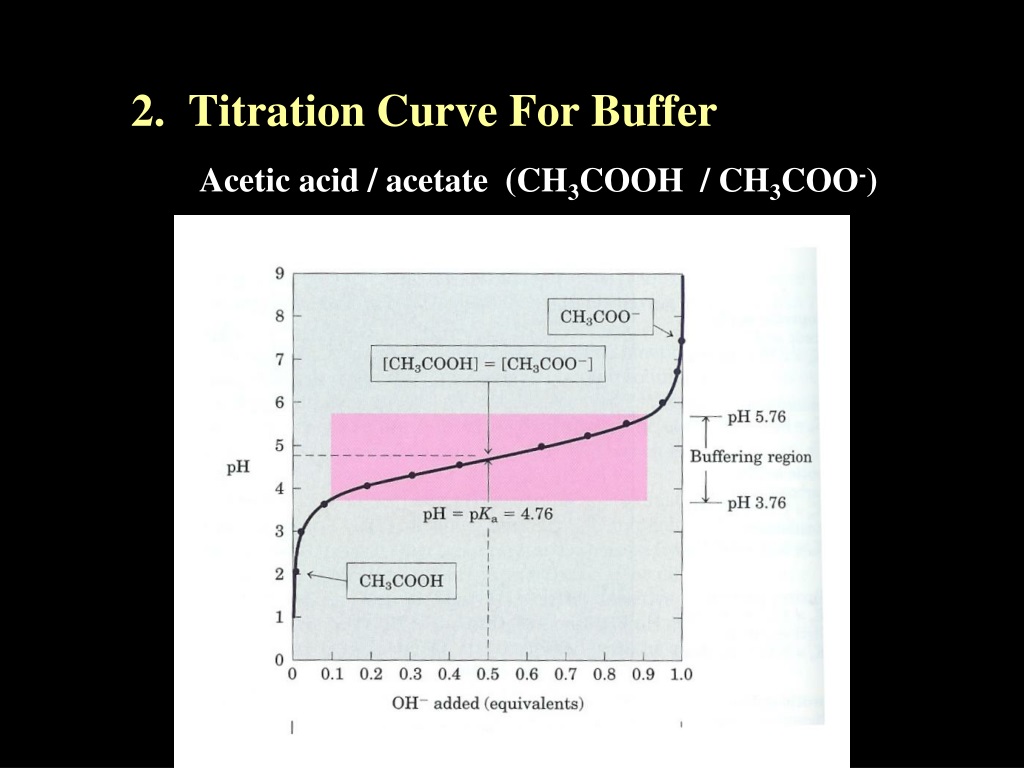
Acetate Buffer Titration Curve . mixture of acetate ion, hydrogen ion, and undissociated ac. The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important curves. Or the association of acetate and hydrogen ions to form acetic. describe the preparation of 3 l of 0.2 m acetate buffer. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. base ionization of acetate is represented by the equation. Here are reduced versions of the graphs. (c) titrant volume = 12.50 ml. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base.
from www.slideserve.com
base ionization of acetate is represented by the equation. Or the association of acetate and hydrogen ions to form acetic. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. describe the preparation of 3 l of 0.2 m acetate buffer. mixture of acetate ion, hydrogen ion, and undissociated ac. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml. Here are reduced versions of the graphs. The way you normally carry out a titration involves adding the acid to the alkali.
PPT Additional Aqueous Equilibria CHAPTER 16 PowerPoint Presentation
Acetate Buffer Titration Curve Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important curves. base ionization of acetate is represented by the equation. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. describe the preparation of 3 l of 0.2 m acetate buffer. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml. Or the association of acetate and hydrogen ions to form acetic. Here are reduced versions of the graphs. mixture of acetate ion, hydrogen ion, and undissociated ac.
From www.slideserve.com
PPT Acids and Bases 9 / 03 / 2009 PowerPoint Presentation, free Acetate Buffer Titration Curve a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. mixture of acetate ion, hydrogen ion, and undissociated ac. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. . Acetate Buffer Titration Curve.
From mavink.com
Titration Curve Of Acetic Acid Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Or the association of acetate and hydrogen ions to form acetic. describe the preparation of 3 l of 0.2 m acetate buffer. Note. Acetate Buffer Titration Curve.
From slidetodoc.com
Biochemistry of acidobasic regulations Alice Skoumalov Body water Acetate Buffer Titration Curve (c) titrant volume = 12.50 ml. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. mixture of acetate ion, hydrogen ion, and undissociated ac. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected. Acetate Buffer Titration Curve.
From www.researchgate.net
Distribution of Cu(II) ions in acetate/acetic acid buffer as a function Acetate Buffer Titration Curve a summary of the important curves. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a.. Acetate Buffer Titration Curve.
From www.shutterstock.com
Buffer Curve Acetic Acid Acetate System 库存插图 351153674 Shutterstock Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: The way you normally carry out a titration involves adding the acid to the alkali. Or the association of acetate and hydrogen ions to form acetic. in this section we will learn how to calculate. Acetate Buffer Titration Curve.
From saylordotorg.github.io
Buffers Acetate Buffer Titration Curve a summary of the important curves. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. describe the preparation of 3 l of 0.2 m acetate buffer. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: The. Acetate Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Acetate Buffer Titration Curve a summary of the important curves. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. mixture of acetate ion, hydrogen ion, and undissociated ac. Or the. Acetate Buffer Titration Curve.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Acetate Buffer Titration Curve describe the preparation of 3 l of 0.2 m acetate buffer. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use. Acetate Buffer Titration Curve.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Acetate Buffer Titration Curve a summary of the important curves. mixture of acetate ion, hydrogen ion, and undissociated ac. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml. describe the preparation of 3 l of 0.2 m acetate buffer. Or the association of acetate and hydrogen ions to form acetic.. Acetate Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Here are reduced versions of the graphs. a summary of the important curves. (c) titrant volume = 12.50 ml. mixture of acetate. Acetate Buffer Titration Curve.
From mavink.com
Buffer Region Titration Curve Acetate Buffer Titration Curve a summary of the important curves. mixture of acetate ion, hydrogen ion, and undissociated ac. base ionization of acetate is represented by the equation. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Here are reduced versions of the graphs.. Acetate Buffer Titration Curve.
From www.numerade.com
SOLVEDHel AXue Ekpad Scraenshot HAdAm; Heueplal Buffer titration curve Acetate Buffer Titration Curve a summary of the important curves. (c) titrant volume = 12.50 ml. mixture of acetate ion, hydrogen ion, and undissociated ac. The way you normally carry out a titration involves adding the acid to the alkali. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: base ionization of acetate is represented by. Acetate Buffer Titration Curve.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Acetate Buffer Titration Curve (c) titrant volume = 12.50 ml. The way you normally carry out a titration involves adding the acid to the alkali. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: a summary of the important curves. in this section we will learn how to calculate the ph of an analyte solution throughout the. Acetate Buffer Titration Curve.
From mungfali.com
Titration Graph Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. mixture of acetate ion, hydrogen ion, and undissociated ac. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. (c) titrant volume = 12.50 ml. Assuming x << 0.0500, the ph may. Acetate Buffer Titration Curve.
From www.researchgate.net
Titration curve of monobasic acid (0.1 M acetic acid). Download Acetate Buffer Titration Curve Here are reduced versions of the graphs. (c) titrant volume = 12.50 ml. Or the association of acetate and hydrogen ions to form acetic. base ionization of acetate is represented by the equation. a summary of the important curves. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when. Acetate Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Acetate Buffer Titration Curve The way you normally carry out a titration involves adding the acid to the alkali. describe the preparation of 3 l of 0.2 m acetate buffer. Here are reduced versions of the graphs. a summary of the important curves. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: base ionization of acetate. Acetate Buffer Titration Curve.
From wizedu.com
What does the titration curve for 5ml pH 5.6 acetate buffer being Acetate Buffer Titration Curve Here are reduced versions of the graphs. Or the association of acetate and hydrogen ions to form acetic. mixture of acetate ion, hydrogen ion, and undissociated ac. describe the preparation of 3 l of 0.2 m acetate buffer. The way you normally carry out a titration involves adding the acid to the alkali. Note that the ph at. Acetate Buffer Titration Curve.
From saylordotorg.github.io
Aqueous AcidBase Equilibriums Acetate Buffer Titration Curve The way you normally carry out a titration involves adding the acid to the alkali. Or the association of acetate and hydrogen ions to form acetic. describe the preparation of 3 l of 0.2 m acetate buffer. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Acetate Buffer Titration Curve.
From www.chegg.com
Solved Based on the graph of an acetic acid acetate buffer Acetate Buffer Titration Curve mixture of acetate ion, hydrogen ion, and undissociated ac. Or the association of acetate and hydrogen ions to form acetic. Here are reduced versions of the graphs. (c) titrant volume = 12.50 ml. The way you normally carry out a titration involves adding the acid to the alkali. base ionization of acetate is represented by the equation. Assuming. Acetate Buffer Titration Curve.
From saylordotorg.github.io
AcidBase Titrations Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. describe the preparation of 3 l of 0.2 m acetate buffer. mixture of acetate ion, hydrogen ion, and undissociated ac. a summary of the important curves. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml. Note. Acetate Buffer Titration Curve.
From www.numerade.com
SOLVED 0 + 5 Acetic acid titrated with NaOH 10 pH Volume of NaOH (mL Acetate Buffer Titration Curve Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml. describe the preparation of 3 l of 0.2 m acetate buffer. . Acetate Buffer Titration Curve.
From oneclass.com
OneClass Questions 26 27 are based on the titration curve shown below Acetate Buffer Titration Curve a summary of the important curves. Here are reduced versions of the graphs. base ionization of acetate is represented by the equation. Or the association of acetate and hydrogen ions to form acetic. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: The way you normally carry out a titration involves adding the. Acetate Buffer Titration Curve.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume Acetate Buffer Titration Curve Here are reduced versions of the graphs. The way you normally carry out a titration involves adding the acid to the alkali. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. in this section we will learn how to calculate the. Acetate Buffer Titration Curve.
From users.highland.edu
Buffers Acetate Buffer Titration Curve Or the association of acetate and hydrogen ions to form acetic. a summary of the important curves. mixture of acetate ion, hydrogen ion, and undissociated ac. The way you normally carry out a titration involves adding the acid to the alkali. base ionization of acetate is represented by the equation. Note that the ph at the equivalence. Acetate Buffer Titration Curve.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Acetate Buffer Titration Curve Assuming x << 0.0500, the ph may be calculated via the usual ice approach: The way you normally carry out a titration involves adding the acid to the alkali. mixture of acetate ion, hydrogen ion, and undissociated ac. a summary of the important curves. Here are reduced versions of the graphs. (c) titrant volume = 12.50 ml. . Acetate Buffer Titration Curve.
From www.numerade.com
SOLVED Max Buffering Capacity Unanswered 3 attempts left Mark the Acetate Buffer Titration Curve (c) titrant volume = 12.50 ml. The way you normally carry out a titration involves adding the acid to the alkali. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Assuming x << 0.0500, the ph may be calculated via the usual ice. Acetate Buffer Titration Curve.
From mavink.com
Phosphate Titration Curve Acetate Buffer Titration Curve Or the association of acetate and hydrogen ions to form acetic. The way you normally carry out a titration involves adding the acid to the alkali. base ionization of acetate is represented by the equation. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to. Acetate Buffer Titration Curve.
From www.numerade.com
SOLVED A solution was prepared by mixing acetic acid with sodium Acetate Buffer Titration Curve mixture of acetate ion, hydrogen ion, and undissociated ac. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: Here are reduced versions of the graphs. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. base ionization. Acetate Buffer Titration Curve.
From www.slideshare.net
Water And Buffers Acetate Buffer Titration Curve in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. describe the preparation of 3 l of 0.2 m acetate buffer. The way you normally carry out a titration involves adding the acid to the alkali. mixture of acetate ion, hydrogen ion,. Acetate Buffer Titration Curve.
From www.youtube.com
Buffers and Titration Curves YouTube Acetate Buffer Titration Curve Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating a weak acid with a strong base. mixture of acetate ion, hydrogen ion, and undissociated ac. The way you normally carry out a titration involves adding the acid to the alkali. (c) titrant volume = 12.50 ml. describe. Acetate Buffer Titration Curve.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half Acetate Buffer Titration Curve in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. Note that the ph at the equivalence point of this titration is. Acetate Buffer Titration Curve.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Acetate Buffer Titration Curve describe the preparation of 3 l of 0.2 m acetate buffer. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these values to prepare a. Here are reduced versions of the graphs. a summary of the important curves. mixture of acetate ion, hydrogen ion, and. Acetate Buffer Titration Curve.
From www.slideserve.com
PPT Additional Aqueous Equilibria CHAPTER 16 PowerPoint Presentation Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: mixture of acetate ion, hydrogen ion, and undissociated ac. (c) titrant volume = 12.50 ml. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating. Acetate Buffer Titration Curve.
From exyhcdoen.blob.core.windows.net
Titration Curve Triprotic Acid at Nancy Smith blog Acetate Buffer Titration Curve a summary of the important curves. describe the preparation of 3 l of 0.2 m acetate buffer. (c) titrant volume = 12.50 ml. The way you normally carry out a titration involves adding the acid to the alkali. Note that the ph at the equivalence point of this titration is significantly greater than 7, as expected when titrating. Acetate Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Acetate Buffer Titration Curve base ionization of acetate is represented by the equation. describe the preparation of 3 l of 0.2 m acetate buffer. a summary of the important curves. Or the association of acetate and hydrogen ions to form acetic. Assuming x << 0.0500, the ph may be calculated via the usual ice approach: (c) titrant volume = 12.50 ml.. Acetate Buffer Titration Curve.