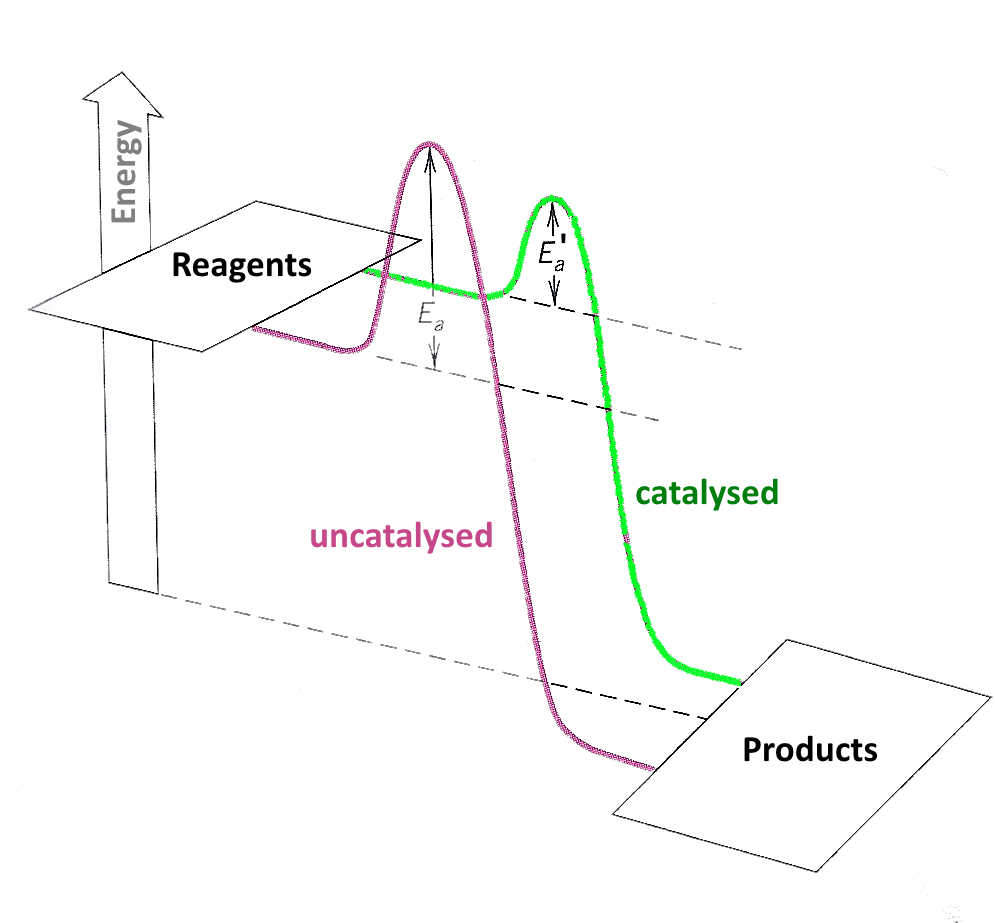
Catalyst Rate Of Reaction Activation Energy . In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Solution activation energies are calculated by. Describe the similarities and differences between the three principal. Reactants often get activation energy from heat,. This page describes and explains the way that adding a catalyst affects the rate of a reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction.
from www.chim.lu
This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Reactants often get activation energy from heat,. What are catalysts, and how do they work in terms altering the parameters of a reaction? In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. This page explains how adding a catalyst affects the rate of a reaction. Solution activation energies are calculated by. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below.
Activation energy and catalysis
Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. Describe the similarities and differences between the three principal. This page explains how adding a catalyst affects the rate of a reaction. Solution activation energies are calculated by. Estimate the activation energy for each process, and identify which one involves a catalyst. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalyst Rate Of Reaction Activation Energy Solution activation energies are calculated by. Reactants often get activation energy from heat,. Describe the similarities and differences between the three principal. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity with. Catalyst Rate Of Reaction Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Rate Of Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes that you are already familiar with basic ideas about the collision theory of reaction. Solution activation. Catalyst Rate Of Reaction Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Catalyst Rate Of Reaction Activation Energy Solution activation energies are calculated by. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a. Catalyst Rate Of Reaction Activation Energy.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Catalyst Rate Of Reaction Activation Energy Estimate the activation energy for each process, and identify which one involves a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Reactants often get activation energy from heat,. Describe the similarities and differences between. Catalyst Rate Of Reaction Activation Energy.
From as-bio-and-chem.blogspot.com
Bio+Chem Notes. ^^ Recapping Rates of Reaction Catalyst Rate Of Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes. Catalyst Rate Of Reaction Activation Energy.
From millerdidettioners.blogspot.com
How Does Particle Size Affect Reaction Rate Miller Didettioners Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. What are catalysts, and how do they work in terms altering the. Catalyst Rate Of Reaction Activation Energy.
From www.youtube.com
Catalyst Affects Reaction Rate Energy Diagram with a Catalyst YouTube Catalyst Rate Of Reaction Activation Energy In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. This page describes and explains the way that adding a catalyst affects the. Catalyst Rate Of Reaction Activation Energy.
From www.youtube.com
Relationship between activation energy and reaction rate Reaction Catalyst Rate Of Reaction Activation Energy It assumes that you are already familiar with basic ideas about the collision theory of reaction. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. Estimate the activation. Catalyst Rate Of Reaction Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Rate Of Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Solution activation energies are calculated by. What are catalysts, and how do they work in terms altering the parameters of a reaction? This page explains. Catalyst Rate Of Reaction Activation Energy.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Catalyst Rate Of Reaction Activation Energy This page explains how adding a catalyst affects the rate of a reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction?. Catalyst Rate Of Reaction Activation Energy.
From philschatz.com
Catalysis · Chemistry Catalyst Rate Of Reaction Activation Energy It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown. Catalyst Rate Of Reaction Activation Energy.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Rate Of Reaction Activation Energy In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page explains how adding a catalyst affects the rate of a reaction. Reactants often get activation energy from heat,.. Catalyst Rate Of Reaction Activation Energy.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Rate Of Reaction Activation Energy Solution activation energies are calculated by. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the. Catalyst Rate Of Reaction Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Rate Of Reaction Activation Energy This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Solution activation energies are calculated by. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts increase the rates. Catalyst Rate Of Reaction Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Rate Of Reaction Activation Energy It assumes that you are already familiar with basic ideas about the collision theory of reaction. Solution activation energies are calculated by. Estimate the activation energy for each process, and identify which one involves a catalyst. This page describes and explains the way that adding a catalyst affects the rate of a reaction. In chemistry and physics, activation energy is. Catalyst Rate Of Reaction Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Rate Of Reaction Activation Energy What are catalysts, and how do they work in terms altering the parameters of a reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. In chemistry and. Catalyst Rate Of Reaction Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyst Rate Of Reaction Activation Energy In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? Solution activation energies are calculated by. In the case of. Catalyst Rate Of Reaction Activation Energy.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalyst Rate Of Reaction Activation Energy What are catalysts, and how do they work in terms altering the parameters of a reaction? Solution activation energies are calculated by. This page explains how adding a catalyst affects the rate of a reaction. Describe the similarities and differences between the three principal. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation. Catalyst Rate Of Reaction Activation Energy.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. Reactants often get activation energy from heat,. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. What are catalysts, and how do they work in terms altering the. Catalyst Rate Of Reaction Activation Energy.
From slideplayer.com
Catalysts Rates of Reactions. ppt download Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Describe the similarities and differences between the three principal. Estimate the activation energy for each process, and identify which. Catalyst Rate Of Reaction Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyst Rate Of Reaction Activation Energy Describe the similarities and differences between the three principal. This page describes and explains the way that adding a catalyst affects the rate of a reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate,. Catalyst Rate Of Reaction Activation Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Rate Of Reaction Activation Energy Solution activation energies are calculated by. Estimate the activation energy for each process, and identify which one involves a catalyst. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. What are catalysts, and how do they work in terms altering the parameters of a reaction?. Catalyst Rate Of Reaction Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst Rate Of Reaction Activation Energy It assumes that you are already familiar with basic ideas about the collision theory of reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often. Catalyst Rate Of Reaction Activation Energy.
From www.chim.lu
Activation energy and catalysis Catalyst Rate Of Reaction Activation Energy It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. This page explains how adding a catalyst affects the rate of a reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between the three principal. Solution activation energies are calculated by. What. Catalyst Rate Of Reaction Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyst Rate Of Reaction Activation Energy It assumes that you are already familiar with basic ideas about the collision theory of reaction. Solution activation energies are calculated by. What are catalysts, and how do they work in terms altering the parameters of a reaction? It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Reactants often get activation energy. Catalyst Rate Of Reaction Activation Energy.
From infinitylearn.com
A catalyst increases the rate of reaction by Sri Chaitanya Infinity Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the collision theory of reaction. What are catalysts, and. Catalyst Rate Of Reaction Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. Estimate the activation energy for each process, and identify which one involves a catalyst. Describe the similarities and differences between. Catalyst Rate Of Reaction Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Rate Of Reaction Activation Energy In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes familiarity with basic concepts in the collision theory of reaction rates, and with the maxwell. What are catalysts, and how do they work in terms altering the parameters of a reaction? Solution activation energies. Catalyst Rate Of Reaction Activation Energy.
From 2012books.lardbucket.org
Catalysis Catalyst Rate Of Reaction Activation Energy Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. This page describes and explains the way that adding a catalyst affects the. Catalyst Rate Of Reaction Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Catalyst Rate Of Reaction Activation Energy This page explains how adding a catalyst affects the rate of a reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. Reactants often get activation energy from heat,. This page describes and explains the way that adding a catalyst affects the rate of a. Catalyst Rate Of Reaction Activation Energy.
From courses.lumenlearning.com
Catalysis Chemistry Catalyst Rate Of Reaction Activation Energy It assumes that you are already familiar with basic ideas about the collision theory of reaction. Estimate the activation energy for each process, and identify which one involves a catalyst. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to overcome. It assumes familiarity with basic concepts. Catalyst Rate Of Reaction Activation Energy.
From www.thesciencehive.co.uk
Reaction Rates* — the science sauce Catalyst Rate Of Reaction Activation Energy Estimate the activation energy for each process, and identify which one involves a catalyst. It assumes that you are already familiar with basic ideas about the collision theory of reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of reaction rates,. Catalyst Rate Of Reaction Activation Energy.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst Rate Of Reaction Activation Energy This page describes and explains the way that adding a catalyst affects the rate of a reaction. Solution activation energies are calculated by. Estimate the activation energy for each process, and identify which one involves a catalyst. This page explains how adding a catalyst affects the rate of a reaction. In the case of a biological reaction, when an enzyme. Catalyst Rate Of Reaction Activation Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Rate Of Reaction Activation Energy This page explains how adding a catalyst affects the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Solution activation energies are calculated by. Estimate the activation energy for each process, and identify which one involves a catalyst. It assumes that you are already familiar with basic ideas. Catalyst Rate Of Reaction Activation Energy.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Catalyst Rate Of Reaction Activation Energy Solution activation energies are calculated by. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy, as shown in the figure below. In the case of a biological reaction, when an enzyme (a form of catalyst) binds. Catalyst Rate Of Reaction Activation Energy.