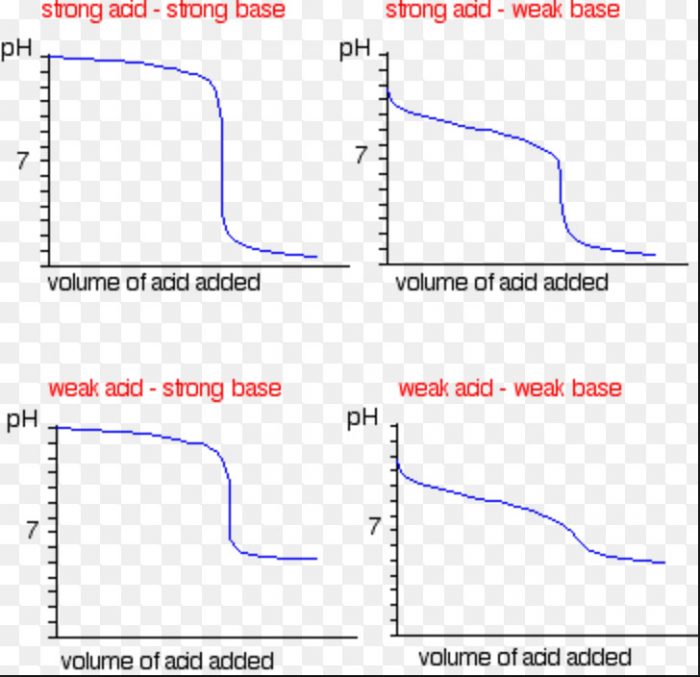
How Many Types Of Titration Are There In Chemistry . Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. It is commonly used in analytical chemistry to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal.
from classnotes.org.in
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal. It is commonly used in analytical chemistry to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
How Many Types Of Titration Are There In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. In an indicator based titration you add another chemical that changes color at the ph equal. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is commonly used in analytical chemistry to.
From mungfali.com
Acid Base Titration Experiment How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. In an indicator based titration you add another chemical that changes color at the ph equal. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is the slow addition of one. How Many Types Of Titration Are There In Chemistry.
From chemistnotes.com
Titration Archives Chemistry Notes How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. In an indicator based titration you add another chemical that changes color at the ph equal. There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent. How Many Types Of Titration Are There In Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration How Many Types Of Titration Are There In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In an indicator based. How Many Types Of Titration Are There In Chemistry.
From www.scribd.com
Titration PDF Titration Chemistry How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is commonly used in analytical chemistry to. In an indicator based titration you add another chemical that changes color. How Many Types Of Titration Are There In Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is the slow addition of one solution of a known. How Many Types Of Titration Are There In Chemistry.
From chemistry.analia-sanchez.net
Titration Notes Chemistry Classes / Ronald Reagan S.H.S. How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal. A titration is a laboratory technique used to precisely measure molar concentration. How Many Types Of Titration Are There In Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple How Many Types Of Titration Are There In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes color at the ph equal. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.. How Many Types Of Titration Are There In Chemistry.
From mungfali.com
Titration Steps How Many Types Of Titration Are There In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is commonly used in analytical chemistry to. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal. Titration, process of chemical. How Many Types Of Titration Are There In Chemistry.
From letitsnowglobe.co.uk
Titration procedure pdf How Many Types Of Titration Are There In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. There are two basic types of acid. How Many Types Of Titration Are There In Chemistry.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. How Many Types Of Titration Are There In Chemistry.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle How Many Types Of Titration Are There In Chemistry In an indicator based titration you add another chemical that changes color at the ph equal. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent. How Many Types Of Titration Are There In Chemistry.
From www.vectorstock.com
Acid base titration experiment and phases Vector Image How Many Types Of Titration Are There In Chemistry There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. It is commonly. How Many Types Of Titration Are There In Chemistry.
From www.dreamstime.com
Titration Vector Illustration. Labeled Educational Chemistry Process How Many Types Of Titration Are There In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and. How Many Types Of Titration Are There In Chemistry.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation How Many Types Of Titration Are There In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. There are two basic types of acid base titrations, indicator and. How Many Types Of Titration Are There In Chemistry.
From www.microlit.com
An Advanced Guide to Titration Microlit How Many Types Of Titration Are There In Chemistry There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. In an indicator based titration you add another chemical that changes color at the ph equal. It is commonly. How Many Types Of Titration Are There In Chemistry.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. It is commonly used in analytical chemistry to. In an indicator based titration you add another chemical that changes color at the ph equal. Titration, process of chemical. How Many Types Of Titration Are There In Chemistry.
From pressbooks.bccampus.ca
8.7 AcidBase Titrations Chemistry for Chemical Engineers How Many Types Of Titration Are There In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. In an indicator based titration you add another chemical that changes. How Many Types Of Titration Are There In Chemistry.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. In an indicator based titration you add another chemical that changes color at the ph equal. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a. How Many Types Of Titration Are There In Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators How Many Types Of Titration Are There In Chemistry In an indicator based titration you add another chemical that changes color at the ph equal. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown. How Many Types Of Titration Are There In Chemistry.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube How Many Types Of Titration Are There In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. In an indicator based titration you add another chemical that changes color at the ph equal.. How Many Types Of Titration Are There In Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. A titration is a laboratory technique used to precisely measure molar. How Many Types Of Titration Are There In Chemistry.
From ask.pharmaguideline.com
What is Titration? And how many types are there, please explain How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution. How Many Types Of Titration Are There In Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. How Many Types Of Titration Are There In Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge How Many Types Of Titration Are There In Chemistry Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes. How Many Types Of Titration Are There In Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How Many Types Of Titration Are There In Chemistry Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is commonly used in analytical chemistry to.. How Many Types Of Titration Are There In Chemistry.
From courses.lumenlearning.com
AcidBase Titrations Chemistry How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. A titration is a laboratory technique used to precisely measure molar. How Many Types Of Titration Are There In Chemistry.
From www.studypool.com
SOLUTION Titration and its types Studypool How Many Types Of Titration Are There In Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. How Many Types Of Titration Are There In Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts How Many Types Of Titration Are There In Chemistry There are two basic types of acid base titrations, indicator and potentiometric. It is commonly used in analytical chemistry to. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. In an indicator based titration you add another chemical that changes color at the ph equal. A titration is a. How Many Types Of Titration Are There In Chemistry.
From www.slideserve.com
PPT Principles of Volumetric (Titrimetric) Analysis PowerPoint How Many Types Of Titration Are There In Chemistry In an indicator based titration you add another chemical that changes color at the ph equal. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration. How Many Types Of Titration Are There In Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How Many Types Of Titration Are There In Chemistry There are two basic types of acid base titrations, indicator and potentiometric. It is commonly used in analytical chemistry to. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. In an indicator based titration you add another chemical that changes. How Many Types Of Titration Are There In Chemistry.
From exyuktqch.blob.core.windows.net
Titration Types Chemistry at Gordon Hutchinson blog How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample of an exactly known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. How Many Types Of Titration Are There In Chemistry.
From www.slideserve.com
PPT Titrations. . . PowerPoint Presentation, free download ID5607639 How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. In an indicator based titration you add another chemical that changes color at the ph equal. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one substance present in another. How Many Types Of Titration Are There In Chemistry.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts How Many Types Of Titration Are There In Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. In an indicator based titration you add another chemical that changes color at the ph equal. It is commonly used. How Many Types Of Titration Are There In Chemistry.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube How Many Types Of Titration Are There In Chemistry There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical. How Many Types Of Titration Are There In Chemistry.
From www.slideshare.net
Chemical calculations part 3 titrations How Many Types Of Titration Are There In Chemistry It is commonly used in analytical chemistry to. There are two basic types of acid base titrations, indicator and potentiometric. Titration is a widely used technique to measure the amount of one substance present in another through a chemical reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. How Many Types Of Titration Are There In Chemistry.