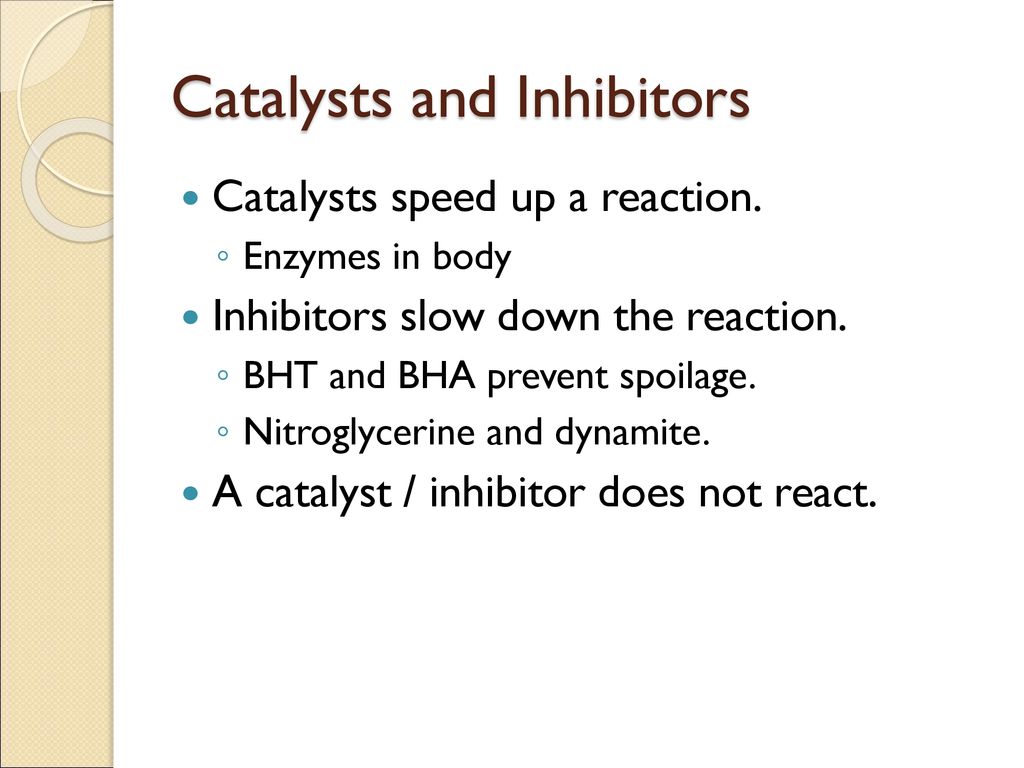
Catalyst And Inhibitor Difference . If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A catalyst is all about energy. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal classes of catalysts. A catalyst lowers the amount of energy needed so that a reaction can happen more easily.
from slideplayer.com
Describe the similarities and differences between the three principal classes of catalysts. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is all about energy. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated.
Introduction to Chemistry Chapter ppt download
Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst is all about energy. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. Describe the similarities and differences between the three principal classes of catalysts. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with. Catalyst And Inhibitor Difference.
From ablogwithadifference.com
Catalyst and Inhibitor The best 5 difference Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. Describe the similarities and differences between the three principal classes of catalysts. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst is all about energy. Inhibition and activation of enzymes via other molecules are other. Catalyst And Inhibitor Difference.
From philschatz.com
Enzymes · Biology Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst is all about energy. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. Describe the similarities and differences between the three principal classes of catalysts. Inhibition and activation of enzymes via other molecules are other important. Catalyst And Inhibitor Difference.
From differencebtw.com
Catalyst vs. Inhibitor Know the Difference Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst is all about energy. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical. Catalyst And Inhibitor Difference.
From pediaa.com
What is the Difference Between Competitive and Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions. Catalyst And Inhibitor Difference.
From thecontentauthority.com
Catalyst vs Inhibitor Which Should You Use In Writing? Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. Describe the similarities and. Catalyst And Inhibitor Difference.
From www.youtube.com
B.7.2 Compare catalysts and biological catalysts (enzymes Catalyst And Inhibitor Difference Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. If you fill a. Catalyst And Inhibitor Difference.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst is all about energy. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. If you fill a room with hydrogen. Catalyst And Inhibitor Difference.
From www.differencebetween.com
Difference Between Catalytic and Stoichiometric Reagents Compare the Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is all about energy. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. While catalysts. Catalyst And Inhibitor Difference.
From slideplayer.com
ppt download Catalyst And Inhibitor Difference Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Describe the similarities and differences between the three principal classes of catalysts. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each. Catalyst And Inhibitor Difference.
From www.youtube.com
Catalyst Promoter and Catalyst Inhibitor Chemistry YouTube Catalyst And Inhibitor Difference A catalyst is all about energy. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Describe the similarities and differences between the. Catalyst And Inhibitor Difference.
From scienceinfo.com
Catalyst and Catalysis Types, Examples, Differences Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A catalyst lowers the amount of energy needed so that a reaction can happen. Catalyst And Inhibitor Difference.
From www.askdifference.com
Catalyst vs. Inhibitor — What’s the Difference? Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is all about energy. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2),. Catalyst And Inhibitor Difference.
From slideplayer.com
Factors Affecting Reaction Rate ppt download Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. A catalyst is all about energy. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of. Catalyst And Inhibitor Difference.
From www.dreamstime.com
Catalyst Competitive Inhibition Active Site Stock Vector Illustration Catalyst And Inhibitor Difference Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is all about energy. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down. Catalyst And Inhibitor Difference.
From www.slideserve.com
PPT Observing and describing chemical reactions PowerPoint Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. A catalyst is all about energy.. Catalyst And Inhibitor Difference.
From slideplayer.com
Equilibrium Chapter ppt download Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. Describe the similarities and differences between the three principal classes of catalysts. Inhibition and activation of enzymes via other molecules. Catalyst And Inhibitor Difference.
From inspiritvr.com
CBSE Class 12 Chemistry Chapter 5 Revision Notes Inspirit Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. A catalyst is all about energy. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst speeds up chemical reactions without being consumed, while an. Catalyst And Inhibitor Difference.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Catalyst And Inhibitor Difference A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. If you fill a room with hydrogen gas (h 2) and oxygen gas (o. Catalyst And Inhibitor Difference.
From www.differencebetween.com
Difference Between Catalyst and Inhibitor Compare the Difference Catalyst And Inhibitor Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Inhibition and activation of enzymes via other molecules are other important ways that enzymes. Catalyst And Inhibitor Difference.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst is all about energy. Describe the similarities and differences between the. Catalyst And Inhibitor Difference.
From www.3ditalian.com
Difference Between Catalyst and Inhibitor Compare the Difference Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst speeds up chemical. Catalyst And Inhibitor Difference.
From 2012books.lardbucket.org
Catalysis Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst is all about energy. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Catalyst And Inhibitor Difference.
From slideplayer.com
Click a hyperlink or folder tab to view the corresponding slides. ppt Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. While catalysts and inhibitors have distinct. Catalyst And Inhibitor Difference.
From www.biologyexams4u.com
Biology Exams 4 U Catalyst And Inhibitor Difference Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with. Catalyst And Inhibitor Difference.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst And Inhibitor Difference Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. A catalyst is all about energy. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst lowers the amount of energy needed so that a reaction can happen. Catalyst And Inhibitor Difference.
From www.youtube.com
Catalysts and Inhibitors YouTube Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. Describe the similarities and differences between the three principal classes of catalysts. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. A. Catalyst And Inhibitor Difference.
From slideplayer.com
Introduction to Chemistry Chapter ppt download Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Describe the similarities and differences between the three principal classes of catalysts. While catalysts and inhibitors have distinct attributes, they. Catalyst And Inhibitor Difference.
From www.youtube.com
Catalyst and Inhibitors YouTube Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst is all about energy. This page looks at the the different. Catalyst And Inhibitor Difference.
From www.jeremyleslie.co.nz
Are you a catalyst or an inhibitor? — Jeremy Leslie Catalyst And Inhibitor Difference A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Inhibition and activation of enzymes via other molecules are other important ways that enzymes are regulated. This page looks at the the. Catalyst And Inhibitor Difference.
From www.learnpick.in
Catalysis PowerPoint Slides LearnPick India Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst is all about energy. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. Inhibition and activation of enzymes via other molecules are other. Catalyst And Inhibitor Difference.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. Describe the similarities and differences between the. Catalyst And Inhibitor Difference.
From pediaa.com
What is the Difference Between Enzyme Activator and Enzyme Inhibitor Catalyst And Inhibitor Difference While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst is all about energy. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. If. Catalyst And Inhibitor Difference.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyst And Inhibitor Difference If you fill a room with hydrogen gas (h 2) and oxygen gas (o 2), very. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst speeds up chemical reactions without being consumed, while an inhibitor slows down or prevents reactions by interfering with the reactants. This page looks at the the different. Catalyst And Inhibitor Difference.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Catalyst And Inhibitor Difference Describe the similarities and differences between the three principal classes of catalysts. A catalyst lowers the amount of energy needed so that a reaction can happen more easily. A catalyst is all about energy. While catalysts and inhibitors have distinct attributes, they both play vital roles in chemical reactions. A catalyst speeds up chemical reactions without being consumed, while an. Catalyst And Inhibitor Difference.