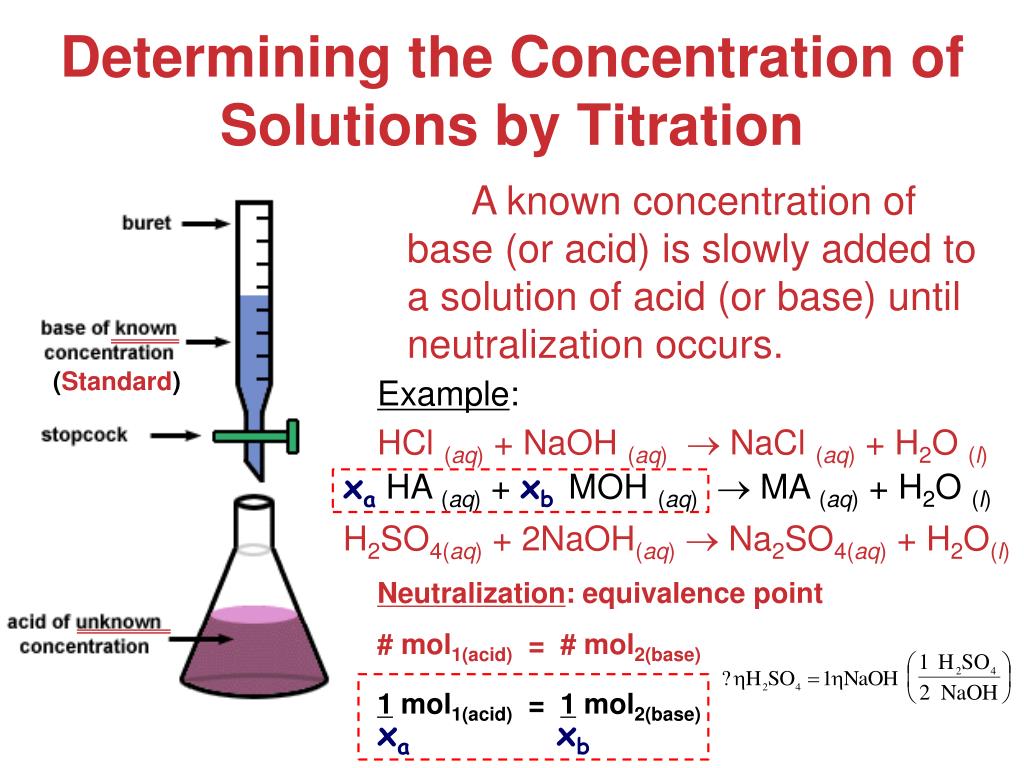
What Is The Conclusion Of Titration . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Depending on the aim of the titration,. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. You may hear or read titration described as an analytical method or technique. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration can also be described as a volumetric method of analysis, because it involves measuring.
from www.slideserve.com
Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. You may hear or read titration described as an analytical method or technique. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This required practical involves using. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. Titration can also be described as a volumetric method of analysis, because it involves measuring. Depending on the aim of the titration,. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali.
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
What Is The Conclusion Of Titration This required practical involves using. You may hear or read titration described as an analytical method or technique. Titration can also be described as a volumetric method of analysis, because it involves measuring. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This required practical involves using. Depending on the aim of the titration,. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to.
From letitsnowglobe.co.uk
Titration procedure pdf What Is The Conclusion Of Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. Titration can also be described as a volumetric method of analysis,. What Is The Conclusion Of Titration.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap What Is The Conclusion Of Titration Depending on the aim of the titration,. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Determination. What Is The Conclusion Of Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 What Is The Conclusion Of Titration Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. This required practical involves using. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. Titration can also be described as a volumetric method of analysis, because it involves measuring. In a titration, the. What Is The Conclusion Of Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps What Is The Conclusion Of Titration In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Depending on the aim of the titration,. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Learn about the titration technique through a practical experiment to determine. What Is The Conclusion Of Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. This. What Is The Conclusion Of Titration.
From www.hanlin.com
IB DP Chemistry SL复习笔记1.2.9 Titrations翰林国际教育 What Is The Conclusion Of Titration Depending on the aim of the titration,. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In a titration, the conclusion is often a simple. What Is The Conclusion Of Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co What Is The Conclusion Of Titration This required practical involves using. Depending on the aim of the titration,. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration can also be described as a volumetric method of analysis, because it involves measuring. In a titration, the conclusion is often a simple statement of. What Is The Conclusion Of Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre What Is The Conclusion Of Titration Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration can also be described as a volumetric method of analysis, because it involves measuring. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is the slow addition of. What Is The Conclusion Of Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is The Conclusion Of Titration Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration can also be described as a volumetric method of analysis, because it involves measuring. Titration (also known as. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download What Is The Conclusion Of Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Depending on the aim of the titration,. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. In a titration, the conclusion is often. What Is The Conclusion Of Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts What Is The Conclusion Of Titration This required practical involves using. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. What Is The Conclusion Of Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Is The Conclusion Of Titration This required practical involves using. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. You may hear or read titration described as an analytical method or technique. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions. What Is The Conclusion Of Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts What Is The Conclusion Of Titration You may hear or read titration described as an analytical method or technique. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. This required practical involves using. The. What Is The Conclusion Of Titration.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts What Is The Conclusion Of Titration Depending on the aim of the titration,. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. This required practical involves using. You may hear or read titration described as an analytical method or technique. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration. What Is The Conclusion Of Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is The Conclusion Of Titration Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration can also be described as a volumetric method of analysis, because it involves measuring. You may hear or read titration described as an analytical method. What Is The Conclusion Of Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors What Is The Conclusion Of Titration Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration can also be described as a volumetric method of analysis, because it involves measuring. Depending on the aim of the titration,. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration is the slow addition of. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. Depending on the aim of the titration,. This required practical involves. What Is The Conclusion Of Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre What Is The Conclusion Of Titration The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. Titration can also be described as a volumetric method of analysis, because it involves measuring. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. What Is The Conclusion Of Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii What Is The Conclusion Of Titration This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. The purpose of the titration is to find the concentration of the hydrochloric acid. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 What Is The Conclusion Of Titration Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Depending on the aim of the titration,. This required practical involves using. You may hear or read titration described as an analytical method or technique. Determination of the reacting volumes of solutions of a strong acid and a. What Is The Conclusion Of Titration.
From slideplayer.com
Titration Of A Strong Base With A Strong Acid Chemistry Lab, Sec ppt What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. The purpose of the titration is to find the concentration of the hydrochloric acid with the help of a base (sodium hydroxide) with a known. This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and. What Is The Conclusion Of Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent What Is The Conclusion Of Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Determination of the reacting volumes of solutions of a strong acid and a strong. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation ID3254207 What Is The Conclusion Of Titration Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is. What Is The Conclusion Of Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME What Is The Conclusion Of Titration Depending on the aim of the titration,. This required practical involves using. You may hear or read titration described as an analytical method or technique. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint What Is The Conclusion Of Titration You may hear or read titration described as an analytical method or technique. Titration can also be described as a volumetric method of analysis, because it involves measuring. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration (also known as titrimetry [1] and volumetric analysis) is. What Is The Conclusion Of Titration.
From theedge.com.hk
Chemistry How To Titration The Edge What Is The Conclusion Of Titration This required practical involves using. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a titration, the conclusion is often a simple statement of the experimentally determined parameter. Titration can also be described as a volumetric method of analysis, because it involves measuring. Learn. What Is The Conclusion Of Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry What Is The Conclusion Of Titration Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Depending on the aim of the titration,. You may hear or read titration described as an analytical method or technique. The. What Is The Conclusion Of Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS What Is The Conclusion Of Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration can also be described as a volumetric method of analysis, because it involves measuring. This required practical involves using. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by. What Is The Conclusion Of Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein What Is The Conclusion Of Titration Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. You may hear or read titration described as an analytical method or technique. Depending on the aim of the titration,. The purpose of the. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. You may hear or read titration described as an analytical method or technique. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. In a titration, the conclusion is often a simple statement of the experimentally determined parameter.. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 What Is The Conclusion Of Titration This required practical involves using. Learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of solutions of acid and alkali. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration can also be described as a volumetric method. What Is The Conclusion Of Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is The Conclusion Of Titration Depending on the aim of the titration,. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Titration can also be described as a volumetric method of analysis, because it involves measuring. You may hear or read titration described as an analytical method or technique. The purpose of the titration is to find. What Is The Conclusion Of Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID What Is The Conclusion Of Titration Titration (also known as titrimetry [1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to. Titration can also be described as a volumetric method of analysis, because it involves measuring. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. The purpose of the titration is to find the. What Is The Conclusion Of Titration.
From mavink.com
Titration Labeled What Is The Conclusion Of Titration Titration can also be described as a volumetric method of analysis, because it involves measuring. Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration. Depending on the aim of the titration,. This required practical involves using. The purpose of the titration is to find the concentration of the hydrochloric acid with the. What Is The Conclusion Of Titration.