Finished Product Analysis . Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Comprehensive review of beer ingredient and finished product analysis. It discusses criteria for selecting. Coverage of organic, inorganic, biochemical, and. Justification of finished product specifications. The suitability of the tests, limits and test methods proposed for the finished product. Contamination of a starting material,. These parameters are assessed and performed for. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Cause variation in the quality of the pharmaceutical product.
from www.wallstreetmojo.com
Justification of finished product specifications. It discusses criteria for selecting. The suitability of the tests, limits and test methods proposed for the finished product. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Coverage of organic, inorganic, biochemical, and. Cause variation in the quality of the pharmaceutical product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Contamination of a starting material,. These parameters are assessed and performed for. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials.
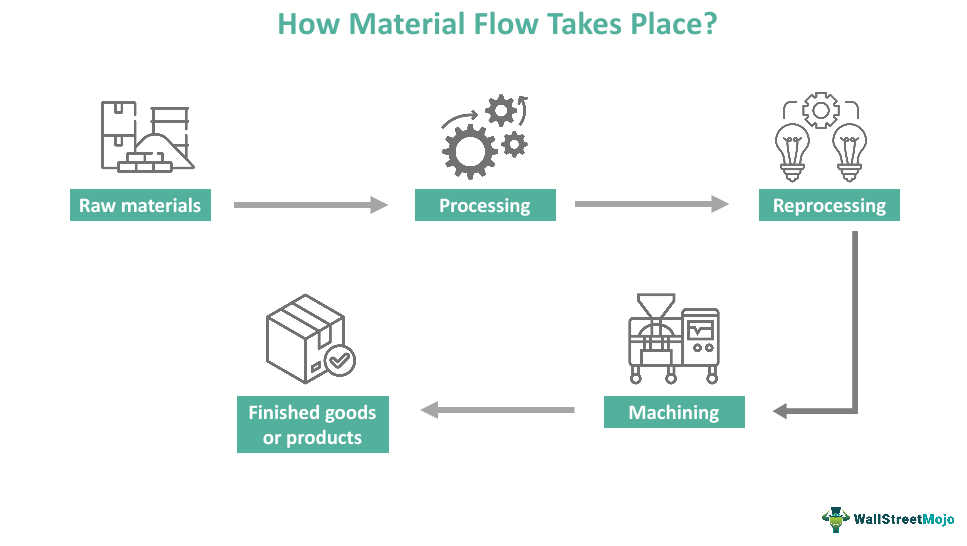
Material Flow What Is It, Types, Examples, Importance
Finished Product Analysis It discusses criteria for selecting. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. It discusses criteria for selecting. Cause variation in the quality of the pharmaceutical product. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Contamination of a starting material,. Coverage of organic, inorganic, biochemical, and. Comprehensive review of beer ingredient and finished product analysis. These parameters are assessed and performed for. Justification of finished product specifications. The suitability of the tests, limits and test methods proposed for the finished product.
From www.pdfprof.com
Binôme STANDUP Chabrerie Cosmetics Finished Product Analysis Comprehensive review of beer ingredient and finished product analysis. Contamination of a starting material,. Justification of finished product specifications. Coverage of organic, inorganic, biochemical, and. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. The suitability of the tests, limits and test methods proposed for the finished product. Finished product analysis. Finished Product Analysis.
From www.researchgate.net
Quality control (QC) test for tablets during process validation (PV Finished Product Analysis Justification of finished product specifications. Comprehensive review of beer ingredient and finished product analysis. These parameters are assessed and performed for. Coverage of organic, inorganic, biochemical, and. The suitability of the tests, limits and test methods proposed for the finished product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Cause. Finished Product Analysis.
From www.slideteam.net
Quality Control Engineering Check Sheet To Assess Finished Product Finished Product Analysis Cause variation in the quality of the pharmaceutical product. These parameters are assessed and performed for. Comprehensive review of beer ingredient and finished product analysis. It discusses criteria for selecting. The suitability of the tests, limits and test methods proposed for the finished product. Contamination of a starting material,. This document summarizes key aspects of raw materials, quality control processes,. Finished Product Analysis.
From pharmablog.in
Testing and Release of In process samples, Semi Finished Products and Finished Product Analysis Coverage of organic, inorganic, biochemical, and. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Comprehensive review of beer ingredient and finished product analysis. It discusses criteria for selecting. These parameters are assessed and performed. Finished Product Analysis.
From www.slideteam.net
Top 7 Product Analysis Templates with Samples and Examples Finished Product Analysis This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. The suitability of the tests, limits and test methods proposed for the finished product. Cause variation in the quality of the pharmaceutical product. Contamination of a. Finished Product Analysis.
From pharmablog.in
Testing and Release of In process samples, Semi Finished Products and Finished Product Analysis By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. These parameters are assessed and performed for. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Cause. Finished Product Analysis.
From template.wps.com
EXCEL of Semi Finished Product Scrap Bill.xlsx WPS Free Templates Finished Product Analysis It discusses criteria for selecting. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Coverage of organic, inorganic, biochemical, and. Cause variation in the quality of the pharmaceutical product. Contamination of a starting material,. Comprehensive review of beer ingredient and finished product analysis. This document summarizes key aspects of raw materials,. Finished Product Analysis.
From snoownews.blogspot.com
Modern Product Specification Template 5 Free Specification Sheet Finished Product Analysis By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Comprehensive review of beer ingredient and finished product analysis. The suitability of the tests, limits and test methods proposed for the finished product. Cause variation in the quality of the pharmaceutical product. Justification of finished product specifications. These parameters are assessed and. Finished Product Analysis.
From pharmaegg.com
SOP for Finished Product Analysis of Sertraline Hydrochloride Tablet By Finished Product Analysis Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Justification of finished product specifications. It discusses criteria for selecting. Coverage of organic, inorganic, biochemical, and. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. These parameters are assessed and performed for. Cause variation in. Finished Product Analysis.
From www.scribd.com
Finished Product Spec Tablet (Pharmacy) Pharmaceutical Sciences Finished Product Analysis Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Cause variation in the quality of the pharmaceutical product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical. Finished Product Analysis.
From www.wordtemplatesonline.net
Free Certificate of Analysis Templates (Guide & Examples) Finished Product Analysis Justification of finished product specifications. Coverage of organic, inorganic, biochemical, and. Comprehensive review of beer ingredient and finished product analysis. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. By conducting thorough product analysis, manufacturers. Finished Product Analysis.
From marfleetanalytical.com
Food Analysis Laboratory Oil Analysis Finished Product Analysis Finished Product Analysis Comprehensive review of beer ingredient and finished product analysis. Justification of finished product specifications. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. These parameters are assessed and performed for. Cause variation in the quality of the pharmaceutical product. Coverage of organic, inorganic, biochemical, and. It discusses criteria for selecting. Finished product analysis. Finished Product Analysis.
From careerfoundry.com
Build A JobReady Data Analyst Portfolio [Best Tips!] Finished Product Analysis Justification of finished product specifications. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Contamination of a starting material,. Comprehensive review of beer ingredient and finished product analysis. Coverage of organic, inorganic, biochemical, and. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and. Finished Product Analysis.
From www.wallstreetmojo.com
Material Flow What Is It, Types, Examples, Importance Finished Product Analysis These parameters are assessed and performed for. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Cause variation in the quality of the pharmaceutical product. Finished product analysis and material testing is the physical and. Finished Product Analysis.
From www.researchgate.net
Finished Product Tests and Observation Download Table Finished Product Analysis Justification of finished product specifications. These parameters are assessed and performed for. The suitability of the tests, limits and test methods proposed for the finished product. Comprehensive review of beer ingredient and finished product analysis. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Contamination of a starting material,. Finished product analysis and. Finished Product Analysis.
From slidesdocs.com
Finished Product Inventory Entry And Exit Details List Excel Template Finished Product Analysis Contamination of a starting material,. The suitability of the tests, limits and test methods proposed for the finished product. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Coverage of organic, inorganic, biochemical, and. Finished. Finished Product Analysis.
From mindmajix.com
Top Product Analyst Interview Questions and Answers in 2023 Finished Product Analysis Justification of finished product specifications. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. The suitability of the tests, limits and test methods proposed for the finished product. Finished product analysis and material testing is. Finished Product Analysis.
From pharmabeej.com
Finished Product Interview Question And Answers Pharmabeej Finished Product Analysis Contamination of a starting material,. The suitability of the tests, limits and test methods proposed for the finished product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Coverage of organic, inorganic, biochemical, and. It discusses criteria for selecting. These parameters are assessed and performed for. Finished product analysis and material. Finished Product Analysis.
From www.durolabs.co
The 6 Stages of a Successful Product Lifecycle Duro Finished Product Analysis Contamination of a starting material,. Comprehensive review of beer ingredient and finished product analysis. Justification of finished product specifications. Cause variation in the quality of the pharmaceutical product. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. These parameters are assessed and performed for. The suitability of the tests, limits and. Finished Product Analysis.
From www.template.net
7+ Certificate of Analysis Template Word , Google docs , Apple pages Finished Product Analysis The suitability of the tests, limits and test methods proposed for the finished product. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Coverage of organic, inorganic, biochemical, and. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. These parameters are assessed and performed. Finished Product Analysis.
From www.pinterest.ph
the sample certificate for manufacturing and manufacturing Finished Product Analysis The suitability of the tests, limits and test methods proposed for the finished product. Contamination of a starting material,. Coverage of organic, inorganic, biochemical, and. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. It discusses criteria for selecting. By conducting thorough product analysis, manufacturers can verify that the final product. Finished Product Analysis.
From help.alisqi.com
Certificate of Analysis AlisQI Help Center Finished Product Analysis By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Comprehensive review of beer ingredient and finished product analysis. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. These parameters are assessed and performed for. The suitability of the tests, limits and test methods proposed. Finished Product Analysis.
From blog.hubspot.com
The Straightforward Guide to Value Chain Analysis [+ Templates] Finished Product Analysis These parameters are assessed and performed for. Justification of finished product specifications. Coverage of organic, inorganic, biochemical, and. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Cause variation in the quality of the pharmaceutical. Finished Product Analysis.
From www.sampletemplates.com
11 Sample Certificate of Analysis Templates to Download Sample Templates Finished Product Analysis Coverage of organic, inorganic, biochemical, and. The suitability of the tests, limits and test methods proposed for the finished product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Contamination of a starting material,. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. These. Finished Product Analysis.
From www.kibrispdr.org
Detail Certificate Of Analysis Template Food Koleksi Nomer 42 Finished Product Analysis It discusses criteria for selecting. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Justification of finished product specifications. Contamination of a starting material,. This document summarizes key aspects of raw materials, quality. Finished Product Analysis.
From www.youtube.com
A Data Analyst to Product Manager Transition Guide YouTube Finished Product Analysis This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. It discusses criteria for selecting. Contamination of a starting material,. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Comprehensive review of beer ingredient and finished product analysis. The suitability of the tests, limits and. Finished Product Analysis.
From www.scribd.com
SOP Storage & Handling of Finished Product PDF Warehouse Finished Product Analysis These parameters are assessed and performed for. Coverage of organic, inorganic, biochemical, and. The suitability of the tests, limits and test methods proposed for the finished product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical. Finished Product Analysis.
From fr.slideshare.net
Textual analysis of finished products Finished Product Analysis Contamination of a starting material,. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Justification of finished product specifications. These parameters are assessed and performed for. Coverage of organic, inorganic, biochemical, and. Comprehensive review of. Finished Product Analysis.
From www.certificateof.com
Merck Certificate of Analysis Certificate Of Finished Product Analysis Cause variation in the quality of the pharmaceutical product. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Contamination of a starting material,. The suitability of the tests, limits and test methods proposed. Finished Product Analysis.
From www.academia.edu
(PDF) Analysis of Temperatures in the Cold Storage of Finished Products Finished Product Analysis It discusses criteria for selecting. The suitability of the tests, limits and test methods proposed for the finished product. Justification of finished product specifications. Cause variation in the quality of the pharmaceutical product. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Coverage of organic, inorganic, biochemical, and. Contamination of a. Finished Product Analysis.
From pharmablog.in
Testing and Release of In process samples, Semi Finished Products and Finished Product Analysis Cause variation in the quality of the pharmaceutical product. Contamination of a starting material,. It discusses criteria for selecting. The suitability of the tests, limits and test methods proposed for the finished product. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Coverage of organic, inorganic, biochemical, and. By conducting thorough. Finished Product Analysis.
From pharmablog.in
Testing and Release of In process samples, Semi Finished Products and Finished Product Analysis Cause variation in the quality of the pharmaceutical product. Comprehensive review of beer ingredient and finished product analysis. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. It discusses criteria for selecting. The. Finished Product Analysis.
From www.honeymanlaboratories.com
Finished Product Analysis and Material Testing Finished Product Analysis Cause variation in the quality of the pharmaceutical product. Justification of finished product specifications. Coverage of organic, inorganic, biochemical, and. The suitability of the tests, limits and test methods proposed for the finished product. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. It discusses criteria for selecting. Comprehensive review of. Finished Product Analysis.
From www.slideteam.net
Product Analysis Example High Temperature Resistance Durability Finished Product Analysis By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance. This document summarizes key aspects of raw materials, quality control processes, and specifications for pharmaceutical products. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Justification of finished product specifications. Cause variation in. Finished Product Analysis.
From www.wordtemplatesonline.net
Free Certificate of Analysis Templates (Guide & Examples) Finished Product Analysis Cause variation in the quality of the pharmaceutical product. Comprehensive review of beer ingredient and finished product analysis. Finished product analysis and material testing is the physical and chemical quality assessment of pharmaceutical items or materials. Coverage of organic, inorganic, biochemical, and. By conducting thorough product analysis, manufacturers can verify that the final product meets the quality standards and performance.. Finished Product Analysis.