Indications For Use . 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Find out the definitions, examples, and tips from emma international experts. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Indications of use = the conditions or reasons for using the device. The difference between intended use and indications for use can be confusing. Here’s how to use both of them for your regulatory strategy. The reasons and the conditions under which the. “indications for use” are about how and when a user will use the device: Intended use = what you say on the label that the device is to be used for.
from www.biomatrixsprx.com
Here’s how to use both of them for your regulatory strategy. The difference between intended use and indications for use can be confusing. “indications for use” are about how and when a user will use the device: Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Intended use = what you say on the label that the device is to be used for. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Indications of use = the conditions or reasons for using the device. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions.
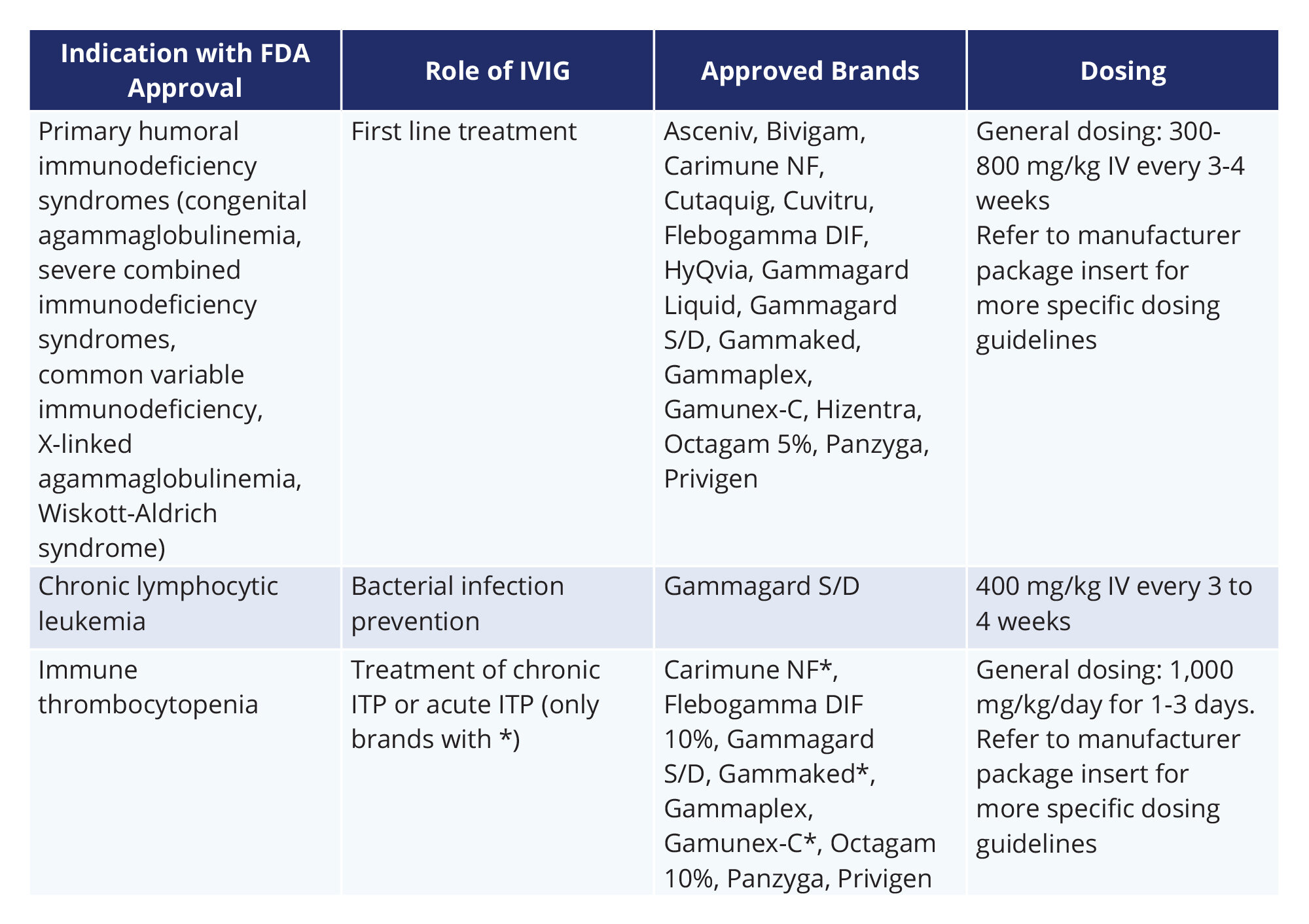
An Overview of Intravenous and Subcutaneous Immunoglobulin (IVIG/SCIG
Indications For Use A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Here’s how to use both of them for your regulatory strategy. Indications of use = the conditions or reasons for using the device. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Find out the definitions, examples, and tips from emma international experts. The reasons and the conditions under which the. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Intended use = what you say on the label that the device is to be used for. “indications for use” are about how and when a user will use the device: A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. The difference between intended use and indications for use can be confusing.
From www.slideserve.com
PPT WOUND DRSSINGS PRINCIPLES & PRACTICE PowerPoint Presentation Indications For Use “indications for use” are about how and when a user will use the device: Indications of use = the conditions or reasons for using the device. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Learn how to determine and communicate these three terms for your medical device product according. Indications For Use.
From www.slideserve.com
PPT Clinical Review PowerPoint Presentation, free download ID2684407 Indications For Use Intended use = what you say on the label that the device is to be used for. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. 'intended use' and the 510 (k). Indications For Use.
From www.yumpu.com
Intended Use/Indications Indications For Use A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Indications of use = the conditions or reasons for using the device. Find out the definitions, examples, and. Indications For Use.
From studyhealthcare.netlify.app
Indications for nasogastric tube insertion Indications For Use Here’s how to use both of them for your regulatory strategy. Indications of use = the conditions or reasons for using the device. Find out the definitions, examples, and tips from emma international experts. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. A 510 (k) is a premarket notification for medical. Indications For Use.
From medicaldeviceacademy.com
Indications for Use inar Indications For Use 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Here’s how to use both of them for your regulatory strategy. Indications of use = the conditions or reasons for using the device. “indications for use” are about how and when a user will use. Indications For Use.
From www.researchgate.net
Indications for echocardiography in acute care settings, evaluated Indications For Use Learn how to determine and communicate these three terms for your medical device product according to fda requirements. The difference between intended use and indications for use can be confusing. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. “indications for use” are about how and when a user will. Indications For Use.
From www.youtube.com
What's the difference between intended use, indications for use, and Indications For Use Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Intended use = what you say on the label that the device is to be used for. Here’s how to use both of them for your regulatory strategy. The reasons and the conditions under which the. 'intended use' and the 510. Indications For Use.
From clin-r.com
Intended Purpose, Intended Use, and Indications for Use Clin R Indications For Use Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Find out the definitions, examples, and tips from emma international experts. The difference between intended use and indications for use can be confusing.. Indications For Use.
From www.greenlight.guru
The Difference Between Intended Use and Indications of Use (And Why Indications For Use “indications for use” are about how and when a user will use the device: Learn how to determine and communicate these three terms for your medical device product according to fda requirements. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Intended use = what you say on the label that. Indications For Use.
From emmainternational.com
Intended Use vs Indications for Use Understanding the Difference Indications For Use Here’s how to use both of them for your regulatory strategy. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. The reasons and the conditions under which the. Learn how to determine and communicate. Indications For Use.
From www.slideserve.com
PPT MEDICATION ADMINISTRATION Topic 1 PowerPoint Presentation, free Indications For Use Intended use = what you say on the label that the device is to be used for. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Find out the definitions, examples, and tips. Indications For Use.
From docslib.org
Folder Guideline 1 Document Indwelling Urinary Catheter Indications Indications For Use Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Intended use = what you say on the label that the device is to be used. Indications For Use.
From www.urotoday.com
Indication of Catheterization for Intermittent Catheters (IC) Indications For Use Indications of use = the conditions or reasons for using the device. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. “indications for use” are about how and when a user will use the device: A 510 (k) is a premarket notification for medical. Indications For Use.
From www.researchgate.net
Updated FDA approved immune checkpoint inhibitors and their indications Indications For Use Intended use = what you say on the label that the device is to be used for. Here’s how to use both of them for your regulatory strategy. The reasons and the conditions under which the. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. 'intended use' and the 510 (k). Indications For Use.
From studylib.net
What is Prosigna™? Indications for Use Indications For Use Indications of use = the conditions or reasons for using the device. The difference between intended use and indications for use can be confusing. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk. Indications For Use.
From www.slideserve.com
PPT Catheterisation PowerPoint Presentation, free download ID634025 Indications For Use The reasons and the conditions under which the. The difference between intended use and indications for use can be confusing. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to. Indications For Use.
From www.aafp.org
Transfusion of Blood and Blood Products Indications and Complications Indications For Use A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Find out the definitions, examples, and tips from emma international experts. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Learn how to determine and communicate. Indications For Use.
From www.bmj.com
Remdesivir for severe covid19 a clinical practice guideline The BMJ Indications For Use Indications of use = the conditions or reasons for using the device. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Find out the definitions, examples,. Indications For Use.
From docslib.org
Carvedilol (Coreg) Considerations for Use* US/FDA Approved Indication Indications For Use Here’s how to use both of them for your regulatory strategy. Intended use = what you say on the label that the device is to be used for. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. 'intended use' and the 510 (k) fda wants you to be very to the point. Indications For Use.
From www.arthroventions.com
KneeTap Indications For Use Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Intended use = what you say on the label that the device is to be used for. Here’s how to use both of them for your regulatory strategy. 'intended use' and the 510 (k) fda wants you to be very to. Indications For Use.
From jamanetwork.com
Developing Indications for the Use of Sentinel Lymph Node Biopsy and Indications For Use Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Find out the definitions, examples, and tips from emma international experts. Indications of use = the conditions or reasons for using the device. The difference between intended use and indications for use can be confusing. A general description of the disease or. Indications For Use.
From www.researchgate.net
Details of indications of use of the allografts retrieved by the tissue Indications For Use Intended use = what you say on the label that the device is to be used for. The reasons and the conditions under which the. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Find out the definitions, examples, and tips from emma international. Indications For Use.
From www.bmj.com
Indications for anticoagulant and antiplatelet combined therapy The BMJ Indications For Use Find out the definitions, examples, and tips from emma international experts. Indications of use = the conditions or reasons for using the device. The reasons and the conditions under which the. The difference between intended use and indications for use can be confusing. “indications for use” are about how and when a user will use the device: Learn how to. Indications For Use.
From clin-r.com
Intended Purpose, Intended Use, and Indications for Use Clin R Indications For Use Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Indications of use = the conditions or reasons for using the device. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. 'intended use' and the 510 (k) fda wants you to be very to. Indications For Use.
From www.slideserve.com
PPT The Modern Approach to Infection Control PowerPoint Presentation Indications For Use “indications for use” are about how and when a user will use the device: Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Find out the definitions, examples, and tips from emma. Indications For Use.
From www.greenlight.guru
The Difference Between Intended Use and Indications of Use (And Why Indications For Use Find out the definitions, examples, and tips from emma international experts. Indications of use = the conditions or reasons for using the device. “indications for use” are about how and when a user will use the device: A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Intended use = what you say. Indications For Use.
From www.slideserve.com
PPT EZ IO Nursing Competency PowerPoint Presentation, free download Indications For Use Indications of use = the conditions or reasons for using the device. Here’s how to use both of them for your regulatory strategy. Intended use = what you say on the label that the device is to be used for. Find out the definitions, examples, and tips from emma international experts. The difference between intended use and indications for use. Indications For Use.
From www.biomatrixsprx.com
An Overview of Intravenous and Subcutaneous Immunoglobulin (IVIG/SCIG Indications For Use Learn how to determine and communicate these three terms for your medical device product according to fda requirements. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Here’s how to use both of them for your regulatory strategy. Find out the definitions, examples, and tips from emma international experts. Indications. Indications For Use.
From www.consultant360.com
Direct Oral Anticoagulants A User’s Guide Consultant360 Indications For Use 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. Indications of use = the conditions or reasons for using the device. The difference between intended use and indications for use can be confusing. “indications for use” are about how and when a user will. Indications For Use.
From www.elexes.com
Intended use vs Indications for use Understand the difference Indications For Use Here’s how to use both of them for your regulatory strategy. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. Intended use = what you say on the label that the device is to be used for. Learn how to determine and communicate these three terms for your medical device. Indications For Use.
From www.slideserve.com
PPT NE 110 Introduction to NDT & QA/QC PowerPoint Presentation ID Indications For Use Intended use = what you say on the label that the device is to be used for. A 510 (k) is a premarket notification for medical devices that demonstrates substantial equivalence to a predicate device. Here’s how to use both of them for your regulatory strategy. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection. Indications For Use.
From www.slideserve.com
PPT AICD and Pacemaker Update PowerPoint Presentation, free download Indications For Use Here’s how to use both of them for your regulatory strategy. Intended use = what you say on the label that the device is to be used for. Learn how to determine and communicate these three terms for your medical device product according to fda requirements. A general description of the disease or condition the device will diagnose, treat, prevent,. Indications For Use.
From clin-r.com
Intended Purpose, Intended Use, and Indications for Use Clin R Indications For Use The difference between intended use and indications for use can be confusing. Intended use = what you say on the label that the device is to be used for. A general description of the disease or condition the device will diagnose, treat, prevent, cure, or mitigate,. Here’s how to use both of them for your regulatory strategy. “indications for use”. Indications For Use.
From www.researchgate.net
Current and potential future indications for the use of the wearable Indications For Use The difference between intended use and indications for use can be confusing. “indications for use” are about how and when a user will use the device: Indications of use = the conditions or reasons for using the device. Find out the definitions, examples, and tips from emma international experts. The reasons and the conditions under which the. Intended use =. Indications For Use.
From www.slideserve.com
PPT FDA Evaluation of Prescription Tests PowerPoint Indications For Use Find out the definitions, examples, and tips from emma international experts. Food and drug administration approved a new indication for use for wegovy (semaglutide) injection to reduce the risk of. 'intended use' and the 510 (k) fda wants you to be very to the point about your stated intended use on your 510 (k) submissions. A 510 (k) is a. Indications For Use.