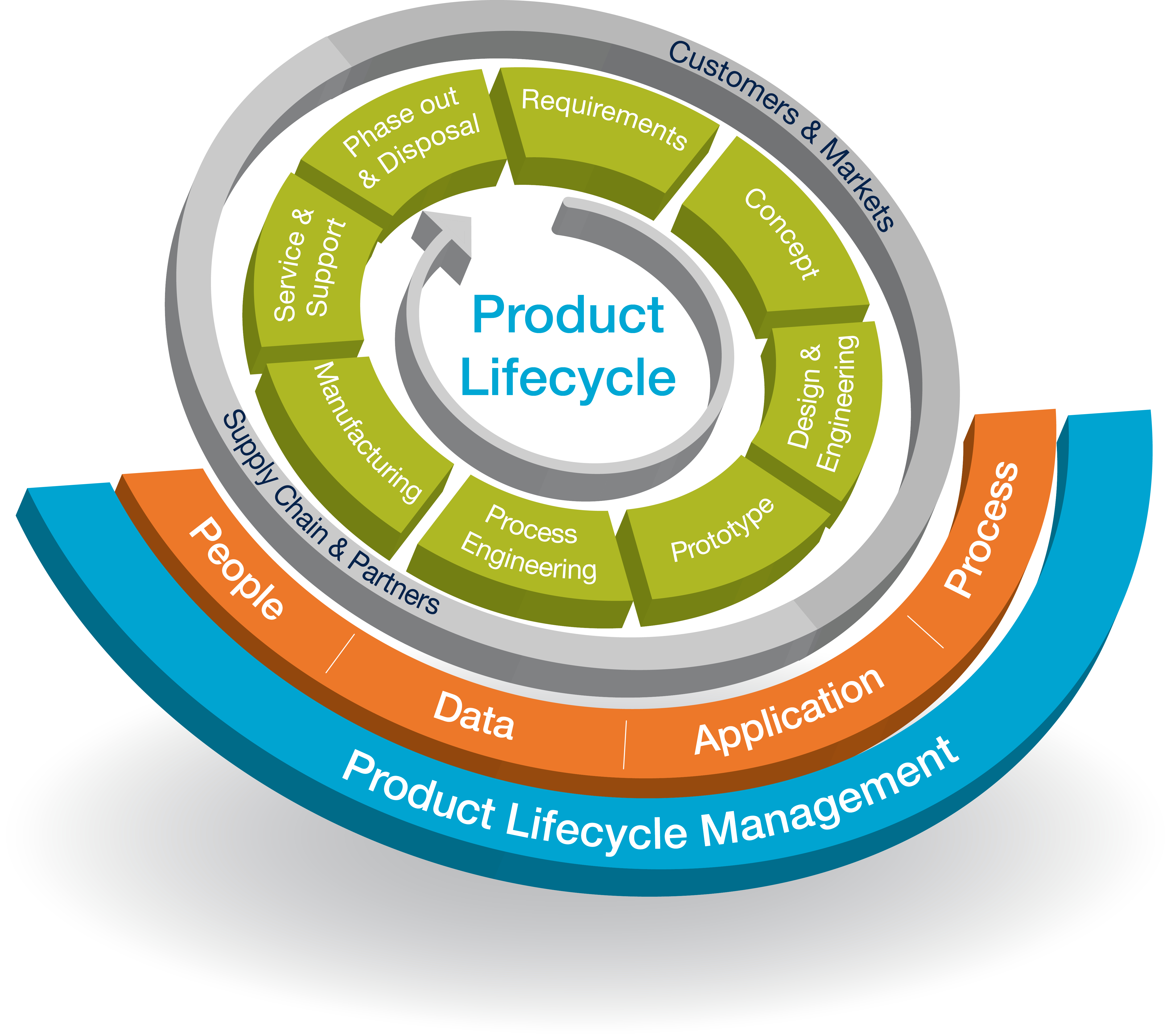
Product Life Cycle Pharma . on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. Journey of drug from ideation to commercialization. And it both complements and. Changes and risk based evaluation. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); From the drug discovery up to its launch. of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. product lifecycle in pharmaceutical industry: Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. there are three distinctive stages of the life cycle of a new drug:
from www.capgemini.com
Journey of drug from ideation to commercialization. Changes and risk based evaluation. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. From the drug discovery up to its launch. there are three distinctive stages of the life cycle of a new drug: product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. And it both complements and. product lifecycle in pharmaceutical industry: pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product.
Product Lifecycle Management
Product Life Cycle Pharma product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. product lifecycle in pharmaceutical industry: of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. Changes and risk based evaluation. there are three distinctive stages of the life cycle of a new drug: on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. From the drug discovery up to its launch. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); And it both complements and. Journey of drug from ideation to commercialization. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product.
From www.valuationlab.com
Life cycle determines risk/reward path We value your business! Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. And it both complements and. there are three distinctive stages of the life cycle of a new drug: this guideline addresses the commercial phase of the product lifecycle (as described in ich. Product Life Cycle Pharma.
From www.smartsheet.com
Ultimate Product Life Cycle Management Guide Smartsheet Product Life Cycle Pharma this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); Changes and risk based evaluation. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. And it both complements and. Journey of drug from ideation to commercialization. product lifecycle management (plm) is the process of managing the entire lifecycle of. Product Life Cycle Pharma.
From cm-plus.co.jp
Pharmaceutical Regulatory Affairs Consulting|CM Plus Corporation Product Life Cycle Pharma product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. product lifecycle in pharmaceutical industry: there are three distinctive stages of the life cycle of a new drug: this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); pharmaceutical product lifecycle management. Product Life Cycle Pharma.
From mungfali.com
Pharmaceutical Product Life Cycle Product Life Cycle Pharma Journey of drug from ideation to commercialization. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. Changes and risk based evaluation. there are three distinctive stages of the life cycle of a new drug: of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. From the drug discovery up to its launch.. Product Life Cycle Pharma.
From www.smartinsights.com
How to use the Product Life Cycle (PLC) marketing model Smart Insights Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. From the drug discovery up to its launch. Changes and risk based evaluation. product lifecycle management (plm). Product Life Cycle Pharma.
From www.capgemini.com
Product Lifecycle Management Product Life Cycle Pharma Changes and risk based evaluation. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. And it both complements and. From the drug discovery up to its launch. of chemistry, manufacturing and controls (. Product Life Cycle Pharma.
From www.pricingsolutions.com
Pricing Research for the Changing Pharma Sector Product Life Cycle Pharma there are three distinctive stages of the life cycle of a new drug: product lifecycle in pharmaceutical industry: product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. this guideline addresses the commercial phase of. Product Life Cycle Pharma.
From ispe.org
Pharmaceutical Product Lifecycle Management ISPE International Product Life Cycle Pharma this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); product lifecycle in pharmaceutical industry: pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. there are three distinctive stages of the life. Product Life Cycle Pharma.
From www.facebook.com
Pharmaceutical Product Life Cycle Management Strategies Pharma Product Life Cycle Pharma Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. From the drug discovery up to its launch. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. And it both complements and. of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. product. Product Life Cycle Pharma.
From www.smartsheet.com
Ultimate Product Life Cycle Management Guide Smartsheet Product Life Cycle Pharma product lifecycle in pharmaceutical industry: From the drug discovery up to its launch. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. there are three distinctive stages of the life cycle of a new drug:. Product Life Cycle Pharma.
From www.thepharmamarketer.com
Product Life Cycle Pharma From the drug discovery up to its launch. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. product lifecycle in pharmaceutical industry: this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); And it both complements and. of chemistry, manufacturing and controls ( cmc) changes across the product. Product Life Cycle Pharma.
From www.investopedia.com
Product Life Cycle Explained Stage and Examples Product Life Cycle Pharma there are three distinctive stages of the life cycle of a new drug: product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); of chemistry, manufacturing and controls ( cmc) changes across the. Product Life Cycle Pharma.
From www.slideteam.net
Pharmaceutical Marketing Life Cycle Management Ppt Powerpoint Product Life Cycle Pharma pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. there are three distinctive stages of the life cycle of a new drug: Journey of drug from ideation to commercialization. From the drug discovery. Product Life Cycle Pharma.
From www.ionos.com
Product life cycle Defining the different product life cycle stages Product Life Cycle Pharma on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. And it both complements and. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); there are three distinctive stages of the life cycle of a new drug: pharmaceutical product lifecycle management is. Product Life Cycle Pharma.
From www.mdpi.com
JOItmC Free FullText Developing a System Dynamic Model for Product Product Life Cycle Pharma From the drug discovery up to its launch. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. product lifecycle in pharmaceutical industry: pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from. Product Life Cycle Pharma.
From webapi.bu.edu
😍 Pharmaceutical industry life cycle stage. PRODUCT LIFE CYCLE Product Life Cycle Pharma on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. From the drug discovery up to its launch. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. . Product Life Cycle Pharma.
From toolbox.eupati.eu
Making a medicine. Step 10 Lifecycle management EUPATI Toolbox Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. this guideline addresses the commercial phase of the product lifecycle (as. Product Life Cycle Pharma.
From www.slideshare.net
PRODUCT LIFE CYCLE....A STUDY ON PHARMA & NONPHARMA EXAMPLE Product Life Cycle Pharma product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. And it both complements and. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. on 20 november 2019, the ich. Product Life Cycle Pharma.
From catie-young.com
How the Pharmaceutical Industry works the product lifecycle part I Product Life Cycle Pharma product lifecycle in pharmaceutical industry: Journey of drug from ideation to commercialization. there are three distinctive stages of the life cycle of a new drug: this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. And. Product Life Cycle Pharma.
From smsrealtime.com
Pharmaceutical Product Life Cycle Management Strategies Smsrealtime Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. And it both complements and. Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical. Product Life Cycle Pharma.
From www.slideteam.net
Pharmaceutical Product Lifecycle Pharmaceutical Development New Product Life Cycle Pharma Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); And it both complements and. Changes and risk based evaluation. product lifecycle in pharmaceutical industry: of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. From the. Product Life Cycle Pharma.
From marketing-insider.eu
Characteristics of the Product Life Cycle Stages and Marketing Implications Product Life Cycle Pharma Changes and risk based evaluation. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. Journey of drug from ideation to commercialization. of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle.. Product Life Cycle Pharma.
From www.smartsheet.com
Ultimate Product Life Cycle Management Guide Smartsheet Product Life Cycle Pharma product lifecycle in pharmaceutical industry: on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. And it both complements and. Journey of drug from ideation to commercialization. From the drug discovery up to its launch. there are three distinctive stages of the life cycle of a new drug: Ich. Product Life Cycle Pharma.
From visual.ly
Product Life Cycle Visual.ly Product Life Cycle Pharma product lifecycle in pharmaceutical industry: product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. From the drug discovery up to its launch. Journey of drug from ideation to commercialization. there are three. Product Life Cycle Pharma.
From www.eleken.co
Growth Stage of Product Life Cycle Things You Need to Know and Implement Product Life Cycle Pharma Journey of drug from ideation to commercialization. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. there are three distinctive stages of the life cycle of a new drug: And it both complements and. product lifecycle management (plm) is the process of managing the entire lifecycle of a. Product Life Cycle Pharma.
From magpazz.blogspot.com
Magpazz Product Life Cycle Pharma From the drug discovery up to its launch. there are three distinctive stages of the life cycle of a new drug: Changes and risk based evaluation. product lifecycle in pharmaceutical industry: on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. product lifecycle management (plm) is the process. Product Life Cycle Pharma.
From vandgrift.com
😊 Pharmaceutical product life cycle. Battling Maturity in the Pharma Product Life Cycle Pharma on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. product lifecycle in pharmaceutical industry: there are three distinctive stages of the life cycle of a new drug: From the. Product Life Cycle Pharma.
From www.researchgate.net
Stages of the medical product life cycle and processes that can be Product Life Cycle Pharma on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. Changes and risk based evaluation. Journey of drug from ideation to commercialization. pharmaceutical product lifecycle management is the process of managing. Product Life Cycle Pharma.
From within3.com
Top pharmaceutical life cycle management strategies Within3 Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. From the drug discovery up to its launch. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. . Product Life Cycle Pharma.
From www.managementguru.net
Product Innovation Management Guru Management Guru Product Life Cycle Pharma this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); From the drug discovery up to its launch. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. . Product Life Cycle Pharma.
From www.emediwrite.com
TOP 10 CHALLENGES IN PHARMACEUTICAL PRODUCT LIFE CYCLE MANAGEMENT Product Life Cycle Pharma Ich q8(r2) and q11 guidelines focus mostly on early stage aspects of the. Changes and risk based evaluation. And it both complements and. product lifecycle in pharmaceutical industry: Journey of drug from ideation to commercialization. on 20 november 2019, the ich assembly endorsed the q12 guideline, “technical and regulatory considerations for pharmaceutical product. From the drug discovery up. Product Life Cycle Pharma.
From www2.deloitte.com
AI in Biopharma Supply Chain Deloitte Insights Product Life Cycle Pharma of chemistry, manufacturing and controls ( cmc) changes across the product lifecycle. From the drug discovery up to its launch. And it both complements and. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); on. Product Life Cycle Pharma.
From www.linkedin.com
Pharma Life Cycle Analysis Why It Is Important Product Life Cycle Pharma there are three distinctive stages of the life cycle of a new drug: And it both complements and. product lifecycle in pharmaceutical industry: From the drug discovery up to its launch. product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. on 20 november 2019, the ich assembly. Product Life Cycle Pharma.
From www.freepik.com
Premium Vector Product lifecycle management or PLM is the process of Product Life Cycle Pharma From the drug discovery up to its launch. product lifecycle in pharmaceutical industry: product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. And it both complements and. on 20 november 2019, the. Product Life Cycle Pharma.
From www.europeanpharmaceuticalreview.com
Pharmaceutical QbD Omnipresence in the product development lifecycle Product Life Cycle Pharma product lifecycle management (plm) is the process of managing the entire lifecycle of a product from its conception,. Journey of drug from ideation to commercialization. pharmaceutical product lifecycle management is the process of managing the entire lifecycle of a product. this guideline addresses the commercial phase of the product lifecycle (as described in ich q10); And it. Product Life Cycle Pharma.