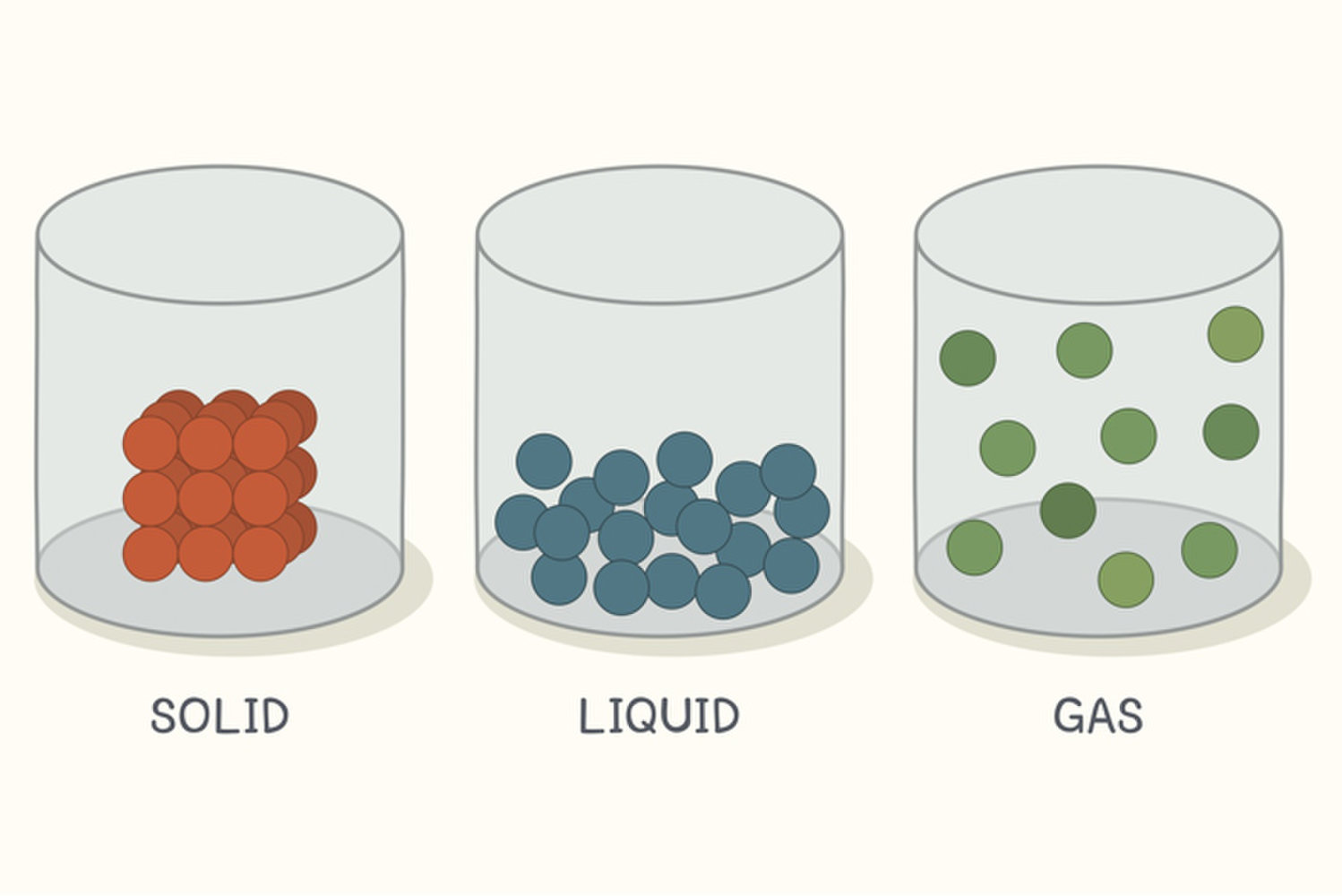
Solid Liquid And Gases In Compressibility . gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Liquid vibrate, move about, and slide past each other. There is no space between. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. This is because the particles in the gas are far apart and there are large. gas vibrate and move freely at high speeds. The thermal expansion of liquids; unlike solids and liquids, gases are highly compressible. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. the atoms, ions, or molecules that make up the solid or liquid are very close together.
from socratic.org
Liquid vibrate, move about, and slide past each other. There is no space between. unlike solids and liquids, gases are highly compressible. This is because the particles in the gas are far apart and there are large. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gas vibrate and move freely at high speeds. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). this model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of liquids; the atoms, ions, or molecules that make up the solid or liquid are very close together.
What are examples of gases, liquids, and solids? Socratic
Solid Liquid And Gases In Compressibility gas vibrate and move freely at high speeds. This is because the particles in the gas are far apart and there are large. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The thermal expansion of liquids; unlike solids and liquids, gases are highly compressible. Liquid vibrate, move about, and slide past each other. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gas vibrate and move freely at high speeds. the atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between.
From www.teachoo.com
Activity to Show Gases can be Compressed but Solids and Liquids cannot Solid Liquid And Gases In Compressibility this model explains the higher density, greater order, and lower compressibility of liquids versus gases; in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. Liquid vibrate, move about, and slide past each other. The thermal expansion of liquids; gas vibrate and move freely at high speeds. gases are. Solid Liquid And Gases In Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid And Gases In Compressibility this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This is because the particles in the gas are far apart and there are large. the atoms, ions, or molecules that make up the solid. Solid Liquid And Gases In Compressibility.
From jordynfersmcguire.blogspot.com
Comparison Between Solid Liquid and Gas Solid Liquid And Gases In Compressibility the atoms, ions, or molecules that make up the solid or liquid are very close together. Liquid vibrate, move about, and slide past each other. This is because the particles in the gas are far apart and there are large. gas vibrate and move freely at high speeds. unlike solids and liquids, gases are highly compressible. . Solid Liquid And Gases In Compressibility.
From brainly.in
difference between solid , liquid and gas on the basis of mass Solid Liquid And Gases In Compressibility the atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between. unlike solids and liquids, gases are highly compressible. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. This is because the particles in the gas are far apart. Solid Liquid And Gases In Compressibility.
From mungfali.com
Solids Liquids Gases Chart Solid Liquid And Gases In Compressibility gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). the atoms, ions, or molecules that make up the solid or liquid are very close together. Liquid vibrate, move about, and slide past each other. gas vibrate and move freely at high speeds. in thermodynamics and fluid mechanics, the. Solid Liquid And Gases In Compressibility.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid And Gases In Compressibility in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. gas vibrate and move freely at high speeds. unlike solids and liquids, gases are highly compressible. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal expansion of liquids; gases are. Solid Liquid And Gases In Compressibility.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Solid Liquid And Gases In Compressibility This is because the particles in the gas are far apart and there are large. The thermal expansion of liquids; There is no space between. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; compressibility is the measure of how much a given volume of matter decreases when placed under pressure. . Solid Liquid And Gases In Compressibility.
From www.slideshare.net
Compressibility of solids , liquids and gases Solid Liquid And Gases In Compressibility unlike solids and liquids, gases are highly compressible. The thermal expansion of liquids; There is no space between. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gas vibrate and move freely at high speeds. Liquid vibrate, move about, and slide past each other. this model explains the. Solid Liquid And Gases In Compressibility.
From www.pinterest.com
Waves travel through solids the fastest because the molecules are Solid Liquid And Gases In Compressibility in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. the atoms, ions, or molecules that make up the solid or liquid are very close together. gas vibrate and move freely at high speeds. compressibility is the measure of how much a given volume of matter decreases when placed. Solid Liquid And Gases In Compressibility.
From www.majordifferences.com
Difference between Solid, Liquid and Gas Table (Solids vs Liquids vs Solid Liquid And Gases In Compressibility in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; This is because the particles in the gas are far apart and there are large. gases are very compressible, so when subjected to high pressures,. Solid Liquid And Gases In Compressibility.
From www.youtube.com
States of Matter Solids, Liquids, Gases & Plasma Chemistry YouTube Solid Liquid And Gases In Compressibility compressibility is the measure of how much a given volume of matter decreases when placed under pressure. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. This is because the particles in the gas are far apart and there are large. the atoms, ions, or molecules that make up. Solid Liquid And Gases In Compressibility.
From www.youtube.com
Activity to show that gases can be compressed more easily than liquids Solid Liquid And Gases In Compressibility gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Liquid vibrate, move about, and slide past each other. There is no space between. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. compressibility is the measure of how much a given. Solid Liquid And Gases In Compressibility.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Solid Liquid And Gases In Compressibility compressibility is the measure of how much a given volume of matter decreases when placed under pressure. unlike solids and liquids, gases are highly compressible. the atoms, ions, or molecules that make up the solid or liquid are very close together. this model explains the higher density, greater order, and lower compressibility of liquids versus gases;. Solid Liquid And Gases In Compressibility.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solid Liquid And Gases In Compressibility this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Liquid vibrate, move about, and slide past each other. the atoms, ions, or molecules that make up the solid or liquid are very close together.. Solid Liquid And Gases In Compressibility.
From www.tec-science.com
Pressure tecscience Solid Liquid And Gases In Compressibility Liquid vibrate, move about, and slide past each other. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). compressibility is the measure of how much a given volume of matter decreases when placed under pressure. This is because the particles in the gas are far apart and there are large.. Solid Liquid And Gases In Compressibility.
From exocprdpx.blob.core.windows.net
Solid Liquid And Gas Particles at Mui Jefferson blog Solid Liquid And Gases In Compressibility gas vibrate and move freely at high speeds. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. The thermal expansion of liquids; the atoms, ions, or molecules that make up the solid or liquid are very close together. in thermodynamics and fluid mechanics, the compressibility (also known as. Solid Liquid And Gases In Compressibility.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Solid Liquid And Gases In Compressibility Liquid vibrate, move about, and slide past each other. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; This is because the particles in the gas are far apart and there are large. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. The thermal. Solid Liquid And Gases In Compressibility.
From exosizztp.blob.core.windows.net
Solids Compressibility at Wilson blog Solid Liquid And Gases In Compressibility This is because the particles in the gas are far apart and there are large. the atoms, ions, or molecules that make up the solid or liquid are very close together. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gas vibrate and move freely at high speeds. Liquid. Solid Liquid And Gases In Compressibility.
From learningmagichazel.z13.web.core.windows.net
Solid Liquid And Gas Lesson Solid Liquid And Gases In Compressibility There is no space between. gas vibrate and move freely at high speeds. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Liquid vibrate, move about, and slide past each other. The thermal expansion of liquids; the atoms, ions, or molecules that make up the solid or liquid are. Solid Liquid And Gases In Compressibility.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Solid Liquid And Gases In Compressibility This is because the particles in the gas are far apart and there are large. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The thermal expansion of liquids; compressibility is the measure of. Solid Liquid And Gases In Compressibility.
From www.slideserve.com
PPT 1) Why is a solid not considered a fluid? PowerPoint Presentation Solid Liquid And Gases In Compressibility gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). This is because the particles in the gas are far apart and there are large. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; unlike solids and liquids, gases are highly compressible. in. Solid Liquid And Gases In Compressibility.
From byjus.com
Activity To show that solids and liquids cannot be compressed but gases Solid Liquid And Gases In Compressibility This is because the particles in the gas are far apart and there are large. The thermal expansion of liquids; There is no space between. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; . Solid Liquid And Gases In Compressibility.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Solid Liquid And Gases In Compressibility There is no space between. the atoms, ions, or molecules that make up the solid or liquid are very close together. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gas vibrate and. Solid Liquid And Gases In Compressibility.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Solid Liquid And Gases In Compressibility in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. unlike solids and liquids, gases are highly compressible. There is no space between. The thermal expansion of liquids; this model explains the higher density, greater order, and lower compressibility of liquids versus gases; gases are very compressible, so when. Solid Liquid And Gases In Compressibility.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Solid Liquid And Gases In Compressibility compressibility is the measure of how much a given volume of matter decreases when placed under pressure. Liquid vibrate, move about, and slide past each other. This is because the particles in the gas are far apart and there are large. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; The thermal. Solid Liquid And Gases In Compressibility.
From www.youtube.com
Lec 8 Some typical properties of gases like density, pressure Solid Liquid And Gases In Compressibility the atoms, ions, or molecules that make up the solid or liquid are very close together. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. This is because the particles in the gas are far apart and there are large. gas vibrate and move freely at high speeds. Liquid. Solid Liquid And Gases In Compressibility.
From brainly.in
With the help of an activity show that gases are more easily Solid Liquid And Gases In Compressibility The thermal expansion of liquids; in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. There is no space between. the atoms, ions, or molecules that make up the solid or liquid are very close together. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly. Solid Liquid And Gases In Compressibility.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Solid Liquid And Gases In Compressibility This is because the particles in the gas are far apart and there are large. There is no space between. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). The thermal expansion of liquids; Liquid vibrate, move about, and slide past each other. the atoms, ions, or molecules that make. Solid Liquid And Gases In Compressibility.
From exosizztp.blob.core.windows.net
Solids Compressibility at Wilson blog Solid Liquid And Gases In Compressibility Liquid vibrate, move about, and slide past each other. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; There is no space between. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). in thermodynamics and fluid mechanics, the compressibility (also known as the. Solid Liquid And Gases In Compressibility.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Solid Liquid And Gases In Compressibility Liquid vibrate, move about, and slide past each other. The thermal expansion of liquids; this model explains the higher density, greater order, and lower compressibility of liquids versus gases; There is no space between. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gases are very compressible, so when. Solid Liquid And Gases In Compressibility.
From www.oxnotes.com
States of Matter Revision Notes IGCSE Chemistry OxNotes GCSE Revision Solid Liquid And Gases In Compressibility unlike solids and liquids, gases are highly compressible. the atoms, ions, or molecules that make up the solid or liquid are very close together. compressibility is the measure of how much a given volume of matter decreases when placed under pressure. gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think. Solid Liquid And Gases In Compressibility.
From www.slideshare.net
Unit 1 Notes Solid Liquid And Gases In Compressibility gases are very compressible, so when subjected to high pressures, their volumes decrease significantly (think boyle’s law!). Liquid vibrate, move about, and slide past each other. the atoms, ions, or molecules that make up the solid or liquid are very close together. unlike solids and liquids, gases are highly compressible. compressibility is the measure of how. Solid Liquid And Gases In Compressibility.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid Liquid And Gases In Compressibility in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. this model explains the higher density, greater order, and lower compressibility of liquids versus gases; Liquid vibrate, move about, and slide past each other. This is because the particles in the gas are far apart and there are large. The thermal. Solid Liquid And Gases In Compressibility.
From brainly.in
Gases are more compressible than solids and liquids explain with a Solid Liquid And Gases In Compressibility Liquid vibrate, move about, and slide past each other. unlike solids and liquids, gases are highly compressible. There is no space between. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. gas vibrate and move freely at high speeds. The thermal expansion of liquids; compressibility is the measure. Solid Liquid And Gases In Compressibility.
From www.onlineworksheet.my.id
Solid Liquid Gas Worksheet Onlineworksheet.my.id Solid Liquid And Gases In Compressibility compressibility is the measure of how much a given volume of matter decreases when placed under pressure. The thermal expansion of liquids; Liquid vibrate, move about, and slide past each other. in thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility[1] or, if the. the atoms, ions, or molecules that make up the. Solid Liquid And Gases In Compressibility.