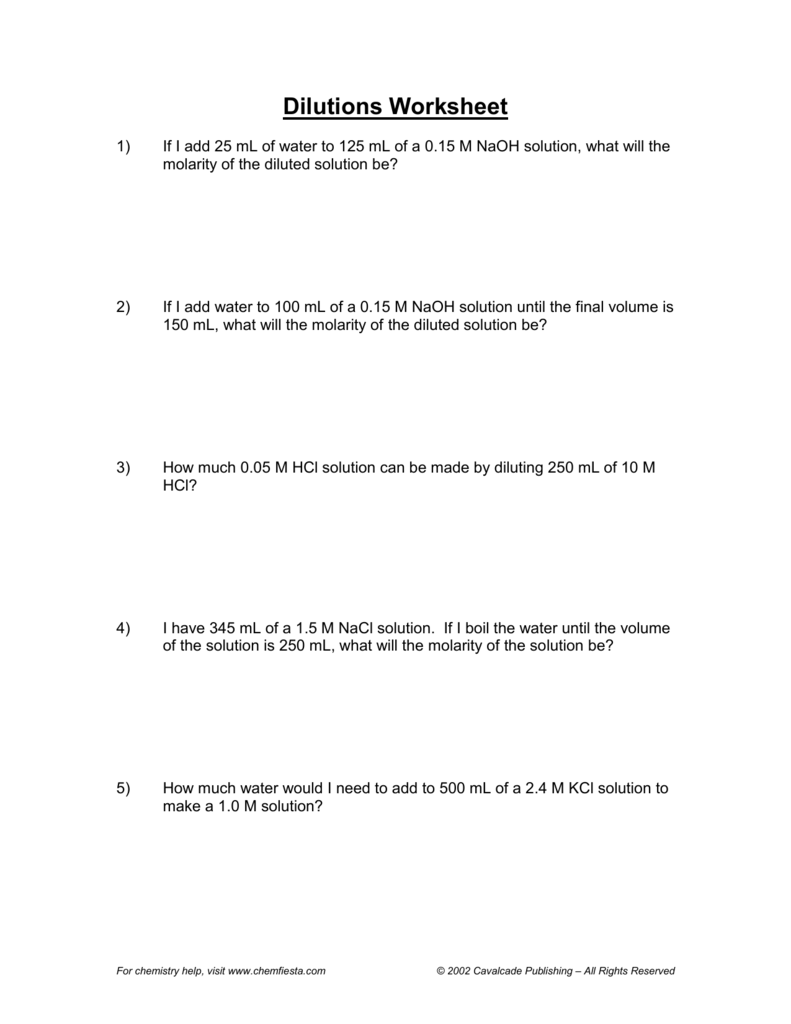
Dilution Chemistry Worksheet . How much of it do you need. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? There’s a bottle of 0.750 m nacl on a shelf. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Check your answers and revise your work on. Molarity and dilution worksheet name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? See examples of calculating molarity, volume and amount of. 1) for each of the following solutions, the number of moles of solute is given, followed by the total.
from studylib.net
1) for each of the following solutions, the number of moles of solute is given, followed by the total. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. How much of it do you need. There’s a bottle of 0.750 m nacl on a shelf. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2.
Dilutions Worksheet
Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. There’s a bottle of 0.750 m nacl on a shelf. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. Molarity and dilution worksheet name: See examples of calculating molarity, volume and amount of. Check your answers and revise your work on. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. How much of it do you need.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Dilution Chemistry Worksheet There’s a bottle of 0.750 m nacl on a shelf. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Molarity and dilution worksheet name: Learn how to solve dilution problems using the equation m1v1 = m2v2. Solve. Dilution Chemistry Worksheet.
From www.liveworksheets.com
Preparing Standard Solution Through Dilution method worksheet Live Dilution Chemistry Worksheet Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. There’s a bottle of 0.750 m nacl on a shelf. How much. Dilution Chemistry Worksheet.
From www.studocu.com
Dilution Worksheet eskandari summer Dilutions Worksheet W 329 Dilution Chemistry Worksheet Molarity and dilution worksheet name: 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Learn how to solve dilution problems using the equation m1v1 = m2v2. There’s a bottle of 0.750 m nacl on a shelf. How much of it do you need. Dilution problems worksheet (m 1 v 1. Dilution Chemistry Worksheet.
From www.studocu.com
Grade 11 Chemistry Dilution Worksheet © John Erickson, 2005 WS15 Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Molarity and dilution worksheet name: See examples of calculating molarity, volume and amount of. There’s a bottle of 0.750 m nacl on a shelf. Solve five problems involving dilutions of acid and salt solutions. Dilution Chemistry Worksheet.
From studylib.net
Dilutions Worksheet 11UChem Dilution Chemistry Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Molarity and dilution worksheet name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Dilution Chemistry Worksheet.
From thekidsworksheet.com
Solutions And Dilutions Worksheet Thekidsworksheet Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add 25 ml of water to 125 ml of a. Dilution Chemistry Worksheet.
From www.docsity.com
Dilution WorkSheet for chemistry Docsity Dilution Chemistry Worksheet If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. See examples of calculating molarity, volume and amount of. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Learn how to. Dilution Chemistry Worksheet.
From pngmusson.blogspot.com
Dilution Calculations Worksheet 2 / Png Musson Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? See examples of calculating molarity, volume and amount of. Molarity and dilution worksheet name: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the. Dilution Chemistry Worksheet.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Dilution Chemistry Worksheet Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution. Dilution Chemistry Worksheet.
From lessonstone.z13.web.core.windows.net
Molarity And Dilution Worksheet Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Learn how to solve dilution problems using the equation m1v1 = m2v2. Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) for each of the following solutions, the number of moles of solute is. Dilution Chemistry Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chemistry Worksheet Check your answers and revise your work on. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Learn how to solve dilution problems using the equation m1v1 = m2v2. There’s a bottle of 0.750 m nacl on a shelf. How much of it. Dilution Chemistry Worksheet.
From printablemagicdrescher.z19.web.core.windows.net
Concentration And Dilution Worksheet Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? How much of it do you need. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? See examples of calculating molarity, volume. Dilution Chemistry Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chemistry Worksheet 1) for each of the following solutions, the number of moles of solute is given, followed by the total. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. How much of it do you need. Molarity and dilution worksheet name: If 455 ml. Dilution Chemistry Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chemistry Worksheet If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? There’s a bottle of 0.750 m nacl on a shelf. Learn how to solve dilution problems using the equation m1v1 =. Dilution Chemistry Worksheet.
From www.chemistrylearner.com
Free Printable Molarity and Dilution Worksheets Dilution Chemistry Worksheet Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) for each of the following. Dilution Chemistry Worksheet.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. How much of it do you need. There’s a bottle of 0.750 m nacl on a shelf. 1) if i add. Dilution Chemistry Worksheet.
From studylib.net
Dilution Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1). Dilution Chemistry Worksheet.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chemistry Worksheet How much of it do you need. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. Molarity and dilution worksheet name: Check your answers and revise your work on. There’s a bottle of 0.750 m nacl on a shelf. Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) for. Dilution Chemistry Worksheet.
From studylib.net
Dilution Worksheet Dilution Chemistry Worksheet See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems using the equation m1v1 = m2v2. Check your answers and revise your work on. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. 1) for each of the following solutions, the number of moles of solute is given, followed by the. Dilution Chemistry Worksheet.
From worksheetkrause.z19.web.core.windows.net
Dilution Problems Chemistry Worksheet Dilution Chemistry Worksheet If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Dilution Chemistry Worksheet.
From studylib.net
Dilutions Worksheet Dilution Chemistry Worksheet Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. How much of it do you need. There’s a bottle of 0.750 m nacl on a shelf. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250. Dilution Chemistry Worksheet.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. There’s a bottle of 0.750 m nacl on a shelf. Check your answers and revise your work on. How much of a 15.0 m stock solution do you need to prepare 250 ml of. Dilution Chemistry Worksheet.
From www.studocu.com
Dilutions Worksheet Dilution problems CHEM 1010 Studocu Dilution Chemistry Worksheet Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? See examples of calculating molarity, volume and amount of. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. Dilution problems. Dilution Chemistry Worksheet.
From studylib.net
Dilutions Worksheet Dilution Chemistry Worksheet If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? See examples of calculating molarity, volume and amount of.. Dilution Chemistry Worksheet.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Learn how to solve dilution problems using the equation m1v1 = m2v2. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml. Dilution Chemistry Worksheet.
From www.chegg.com
Solved Dilution Worksheet 2 Show all work in the spaces Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Dilution Chemistry Worksheet.
From www.chemistryworksheet.com
Dilution Calculation Worksheet With Answers Printable Pdf Download Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? How much of it do you need. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) for each of the following. Dilution Chemistry Worksheet.
From worksheets.clipart-library.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chemistry Worksheet Molarity and dilution worksheet name: There’s a bottle of 0.750 m nacl on a shelf. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Learn how to solve dilution problems using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of. Dilution Chemistry Worksheet.
From studylib.net
Solutions & Dilutions Worksheet Dilution Chemistry Worksheet Dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. There’s a bottle of 0.750 m nacl on a shelf. Molarity and dilution worksheet name: Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. Check your answers. Dilution Chemistry Worksheet.
From afzanituall.blogspot.com
Dilutions Worksheet Dilutions Worksheet Free division worksheets Dilution Chemistry Worksheet See examples of calculating molarity, volume and amount of. If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? How much of it do. Dilution Chemistry Worksheet.
From worksheets.clipart-library.com
Molarity and Dilutions Notes and Worksheet Set by Chemistry Wiz Dilution Chemistry Worksheet How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Check your answers and revise your work on. Learn how to solve dilution problems using the equation m1v1 = m2v2. How much of it do you need. Dilution problems worksheet (m 1 v 1 = m 2 v 2). Dilution Chemistry Worksheet.
From learninglibrarylinton.z21.web.core.windows.net
Dilution Practice Problems Worksheet Answers Dilution Chemistry Worksheet Check your answers and revise your work on. See examples of calculating molarity, volume and amount of. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. How much of it. Dilution Chemistry Worksheet.
From materialzoneschmid.z13.web.core.windows.net
Dilution Worksheet Chemistry Dilution Chemistry Worksheet How much of it do you need. There’s a bottle of 0.750 m nacl on a shelf. Check your answers and revise your work on. See examples of calculating molarity, volume and amount of. 1) for each of the following solutions, the number of moles of solute is given, followed by the total. Molarity and dilution worksheet name: How much. Dilution Chemistry Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chemistry Worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. Check your answers and revise your work on. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Learn how to solve dilution. Dilution Chemistry Worksheet.
From classschoolcole.z21.web.core.windows.net
Molarity By Dilution Worksheet Dilution Chemistry Worksheet If 455 ml of 6.0 m hno3 is diluted to 2.5 l, what. Solve five problems involving dilutions of acid and salt solutions using the equation m1v1 = m2v2. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be?. 1) if i add 25. Dilution Chemistry Worksheet.