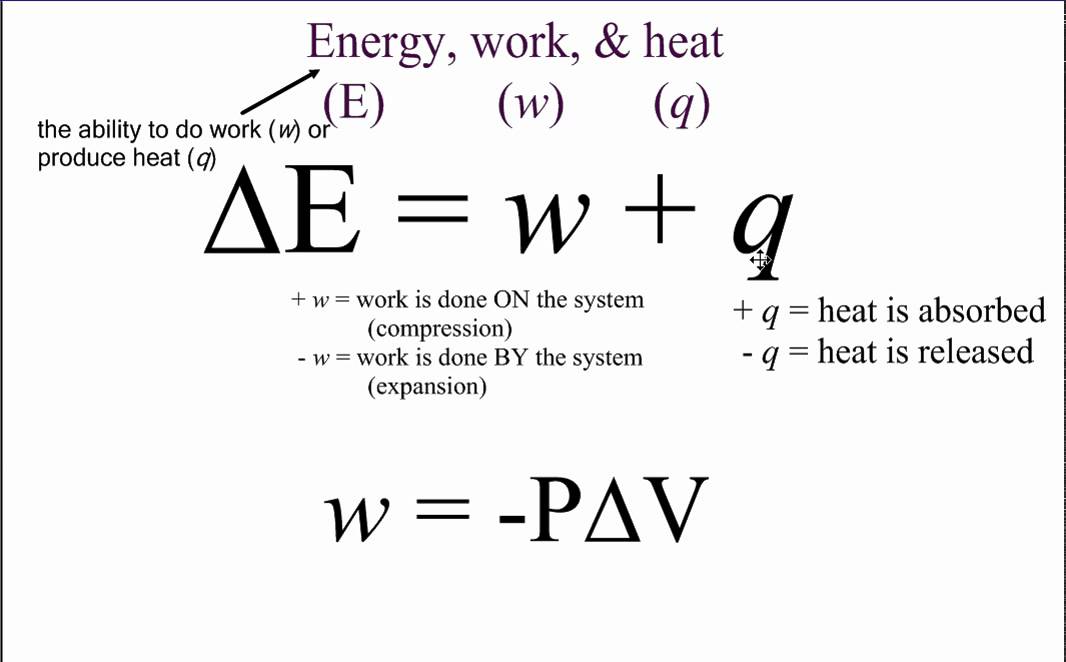
Define Heat And Work . How do they differ from. Work involves an orderly motion of. There are other equivalent definitions. Heat and work are both measured in energy units, so they must both represent energy. The processes are very different. Calculate the work, heat transfer, and internal energy change in a simple process We have already defined work as a force acting through a distance. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. when you think about the molecules, the difference between work and heat is very simple. Thermodynamics deals with the transfer of energy from. thermodynamics, science of the relationship between heat, work, temperature, and energy. heat (q) and work (w) are the two ways to add or remove energy from a system. heat and work. describe the work done by a system, heat transfer between objects, and internal energy change of a system;
from www.youtube.com
describe the work done by a system, heat transfer between objects, and internal energy change of a system; We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. Thermodynamics deals with the transfer of energy from. thermodynamics, science of the relationship between heat, work, temperature, and energy. Work involves an orderly motion of. There are other equivalent definitions. heat (q) and work (w) are the two ways to add or remove energy from a system. when you think about the molecules, the difference between work and heat is very simple. heat and work. We have already defined work as a force acting through a distance.
Energy work and heat YouTube
Define Heat And Work The processes are very different. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. We have already defined work as a force acting through a distance. Work involves an orderly motion of. when you think about the molecules, the difference between work and heat is very simple. Relate the amount of heat to a temperature change. The processes are very different. thermodynamics, science of the relationship between heat, work, temperature, and energy. Calculate the work, heat transfer, and internal energy change in a simple process heat and work. Heat and work are both measured in energy units, so they must both represent energy. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. There are other equivalent definitions. heat (q) and work (w) are the two ways to add or remove energy from a system. Thermodynamics deals with the transfer of energy from. describe the work done by a system, heat transfer between objects, and internal energy change of a system;
From www.youtube.com
Energy work and heat YouTube Define Heat And Work There are other equivalent definitions. thermodynamics, science of the relationship between heat, work, temperature, and energy. Work involves an orderly motion of. when you think about the molecules, the difference between work and heat is very simple. Thermodynamics deals with the transfer of energy from. heat (q) and work (w) are the two ways to add or. Define Heat And Work.
From www.slideserve.com
PPT Chapter 18 Heat, Work, and the First Law of Thermodynamics Define Heat And Work Work involves an orderly motion of. How do they differ from. The processes are very different. We have already defined work as a force acting through a distance. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. Thermodynamics deals with the transfer of energy from. heat and. Define Heat And Work.
From www.youtube.com
Comparison Of Work And Heat YouTube Define Heat And Work Relate the amount of heat to a temperature change. Thermodynamics deals with the transfer of energy from. We have already defined work as a force acting through a distance. when you think about the molecules, the difference between work and heat is very simple. heat (q) and work (w) are the two ways to add or remove energy. Define Heat And Work.
From www.britannica.com
Convection Definition, Examples, Types, & Facts Britannica Define Heat And Work We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. How do they differ from. when you think about the molecules, the difference between work and heat is very simple. heat and work. heat (q) and work (w) are the two ways to add or remove. Define Heat And Work.
From studylib.net
Work and Heat Definitions FL1 Define Heat And Work There are other equivalent definitions. thermodynamics, science of the relationship between heat, work, temperature, and energy. Work involves an orderly motion of. Calculate the work, heat transfer, and internal energy change in a simple process heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. heat and. Define Heat And Work.
From www.engineeringpassion.com
Heat VS Work Concepts Explained Engineering Passion Define Heat And Work Relate the amount of heat to a temperature change. Work involves an orderly motion of. The processes are very different. Heat and work are both measured in energy units, so they must both represent energy. when you think about the molecules, the difference between work and heat is very simple. heat (q) and work (w) are the two. Define Heat And Work.
From www.youtube.com
Heat (w), Work (q), Change in Internal Energy (∆E) Practice Problems Define Heat And Work We have already defined work as a force acting through a distance. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. How do they differ from. heat (q) and work (w) are the two ways to add or remove energy from a system. We’ve seen that the. Define Heat And Work.
From www.slideserve.com
PPT Principles of Chemistry A Molecular Approach , 1st Ed. Nivaldo Define Heat And Work describe the work done by a system, heat transfer between objects, and internal energy change of a system; How do they differ from. Heat and work are both measured in energy units, so they must both represent energy. Calculate the work, heat transfer, and internal energy change in a simple process heat and work. heat is the. Define Heat And Work.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID822227 Define Heat And Work The processes are very different. Heat and work are both measured in energy units, so they must both represent energy. Calculate the work, heat transfer, and internal energy change in a simple process thermodynamics, science of the relationship between heat, work, temperature, and energy. We have already defined work as a force acting through a distance. heat is. Define Heat And Work.
From www.thoughtco.com
Definition and Examples of Heat Energy Define Heat And Work Calculate the work, heat transfer, and internal energy change in a simple process Thermodynamics deals with the transfer of energy from. How do they differ from. heat (q) and work (w) are the two ways to add or remove energy from a system. Heat and work are both measured in energy units, so they must both represent energy. . Define Heat And Work.
From learningdbgoldberg.z21.web.core.windows.net
Explain The Three Methods Of Heat Transfer Define Heat And Work heat (q) and work (w) are the two ways to add or remove energy from a system. heat and work. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. Thermodynamics deals with the transfer of energy from. How do they differ from. when you think. Define Heat And Work.
From www.slideserve.com
PPT Heat Q vs Work W and efficiency PowerPoint Presentation, free Define Heat And Work describe the work done by a system, heat transfer between objects, and internal energy change of a system; Heat and work are both measured in energy units, so they must both represent energy. How do they differ from. Thermodynamics deals with the transfer of energy from. We’ve seen that the internal energy changes with q, which is the net. Define Heat And Work.
From www.slideserve.com
PPT HEAT, WORK AND INTERNAL ENERGY PowerPoint Presentation, free Define Heat And Work The processes are very different. Work involves an orderly motion of. when you think about the molecules, the difference between work and heat is very simple. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. Thermodynamics deals with the transfer of energy from. We’ve seen that the. Define Heat And Work.
From www.slideserve.com
PPT Gravity was application of a fundamental force law (of the 4 Define Heat And Work Relate the amount of heat to a temperature change. thermodynamics, science of the relationship between heat, work, temperature, and energy. There are other equivalent definitions. heat and work. heat (q) and work (w) are the two ways to add or remove energy from a system. Thermodynamics deals with the transfer of energy from. How do they differ. Define Heat And Work.
From study.com
Thermal Energy Definition & Examples Lesson Define Heat And Work How do they differ from. Work involves an orderly motion of. when you think about the molecules, the difference between work and heat is very simple. describe the work done by a system, heat transfer between objects, and internal energy change of a system; We’ve seen that the internal energy changes with q, which is the net heat. Define Heat And Work.
From www.slideserve.com
PPT Q&A_6 10/20/2005(6) JiSheng Chang PowerPoint Presentation ID Define Heat And Work Calculate the work, heat transfer, and internal energy change in a simple process when you think about the molecules, the difference between work and heat is very simple. Thermodynamics deals with the transfer of energy from. heat and work. Relate the amount of heat to a temperature change. Heat and work are both measured in energy units, so. Define Heat And Work.
From physical-phenomena.blogspot.com
Laws of Thermodynamics Define Heat And Work when you think about the molecules, the difference between work and heat is very simple. The processes are very different. thermodynamics, science of the relationship between heat, work, temperature, and energy. Work involves an orderly motion of. How do they differ from. describe the work done by a system, heat transfer between objects, and internal energy change. Define Heat And Work.
From studylib.net
Energy, Work and Heat Define Heat And Work heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. describe the work done by a system, heat transfer between objects, and internal energy change of a system; when you think about the molecules, the difference between work and heat is very simple. How do they differ. Define Heat And Work.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Define Heat And Work heat and work. heat (q) and work (w) are the two ways to add or remove energy from a system. Calculate the work, heat transfer, and internal energy change in a simple process We have already defined work as a force acting through a distance. Relate the amount of heat to a temperature change. thermodynamics, science of. Define Heat And Work.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Define Heat And Work Relate the amount of heat to a temperature change. when you think about the molecules, the difference between work and heat is very simple. The processes are very different. Heat and work are both measured in energy units, so they must both represent energy. Work involves an orderly motion of. We have already defined work as a force acting. Define Heat And Work.
From ceccwqmh.blob.core.windows.net
Induction Cooktop Info at Fatima Smith blog Define Heat And Work The processes are very different. Thermodynamics deals with the transfer of energy from. Calculate the work, heat transfer, and internal energy change in a simple process Work involves an orderly motion of. thermodynamics, science of the relationship between heat, work, temperature, and energy. describe the work done by a system, heat transfer between objects, and internal energy change. Define Heat And Work.
From stock.adobe.com
Heat energy as convection, conduction and radiation, physics science Define Heat And Work thermodynamics, science of the relationship between heat, work, temperature, and energy. There are other equivalent definitions. Calculate the work, heat transfer, and internal energy change in a simple process Work involves an orderly motion of. describe the work done by a system, heat transfer between objects, and internal energy change of a system; when you think about. Define Heat And Work.
From www.youtube.com
31. Heat and Work Differences Complete Concept YouTube Define Heat And Work How do they differ from. when you think about the molecules, the difference between work and heat is very simple. Heat and work are both measured in energy units, so they must both represent energy. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. heat and. Define Heat And Work.
From www.differencebetween.com
Difference Between Work and Heat Compare the Difference Between Define Heat And Work thermodynamics, science of the relationship between heat, work, temperature, and energy. We have already defined work as a force acting through a distance. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. There are other equivalent definitions. when you think about the molecules, the difference between. Define Heat And Work.
From www.youtube.com
Thermodynamics Energy, Work and Heat (Animation) YouTube Define Heat And Work Heat and work are both measured in energy units, so they must both represent energy. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. The processes are very different. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between. Define Heat And Work.
From keydifference.in
9 Best Difference Between Work and Heat Key Difference Define Heat And Work We have already defined work as a force acting through a distance. Calculate the work, heat transfer, and internal energy change in a simple process Work involves an orderly motion of. heat (q) and work (w) are the two ways to add or remove energy from a system. heat is the transfer of thermal energy between systems, while. Define Heat And Work.
From www.youtube.com
First Law of Thermodynamics, Basic Introduction Internal Energy, Heat Define Heat And Work The processes are very different. describe the work done by a system, heat transfer between objects, and internal energy change of a system; when you think about the molecules, the difference between work and heat is very simple. Work involves an orderly motion of. We’ve seen that the internal energy changes with q, which is the net heat. Define Heat And Work.
From www.slideshare.net
Heat and work Define Heat And Work How do they differ from. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. Relate the amount of heat to a temperature change. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. Thermodynamics deals with the. Define Heat And Work.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs Define Heat And Work Heat and work are both measured in energy units, so they must both represent energy. We have already defined work as a force acting through a distance. when you think about the molecules, the difference between work and heat is very simple. The processes are very different. heat is the transfer of thermal energy between systems, while work. Define Heat And Work.
From sciencenotes.org
What Is Temperature? Definition in Science Define Heat And Work There are other equivalent definitions. Relate the amount of heat to a temperature change. Heat and work are both measured in energy units, so they must both represent energy. heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. How do they differ from. thermodynamics, science of the. Define Heat And Work.
From keydifference.in
9 Best Difference Between Work and Heat Key Difference Define Heat And Work Calculate the work, heat transfer, and internal energy change in a simple process heat and work. We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. We have already defined work as a force acting through a distance. The processes are very different. when you think about. Define Heat And Work.
From www.grc.nasa.gov
Heat Transfer Define Heat And Work Heat and work are both measured in energy units, so they must both represent energy. when you think about the molecules, the difference between work and heat is very simple. The processes are very different. There are other equivalent definitions. Calculate the work, heat transfer, and internal energy change in a simple process Work involves an orderly motion of.. Define Heat And Work.
From www.slideserve.com
PPT Work in Thermodynamic Processes PowerPoint Presentation, free Define Heat And Work We’ve seen that the internal energy changes with q, which is the net heat added to the system and w, which. heat and work. There are other equivalent definitions. Work involves an orderly motion of. How do they differ from. thermodynamics, science of the relationship between heat, work, temperature, and energy. The processes are very different. We have. Define Heat And Work.
From www.studocu.com
7 Q4 Science Science Quarter 4 Module 7 HEAT, WORK, and ENERGY Define Heat And Work Thermodynamics deals with the transfer of energy from. Heat and work are both measured in energy units, so they must both represent energy. Calculate the work, heat transfer, and internal energy change in a simple process There are other equivalent definitions. Work involves an orderly motion of. The processes are very different. How do they differ from. thermodynamics, science. Define Heat And Work.
From www.youtube.com
Thermodynamics Sign Convention for Work & Heat YouTube Define Heat And Work Thermodynamics deals with the transfer of energy from. describe the work done by a system, heat transfer between objects, and internal energy change of a system; How do they differ from. Work involves an orderly motion of. Relate the amount of heat to a temperature change. The processes are very different. We have already defined work as a force. Define Heat And Work.