Quality Control Guidelines Pharmaceuticals . all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. this document describes a model for an effective quality management system. It applies to the development and manufacture.
from www.slideteam.net
our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. this document describes a model for an effective quality management system. It applies to the development and manufacture.
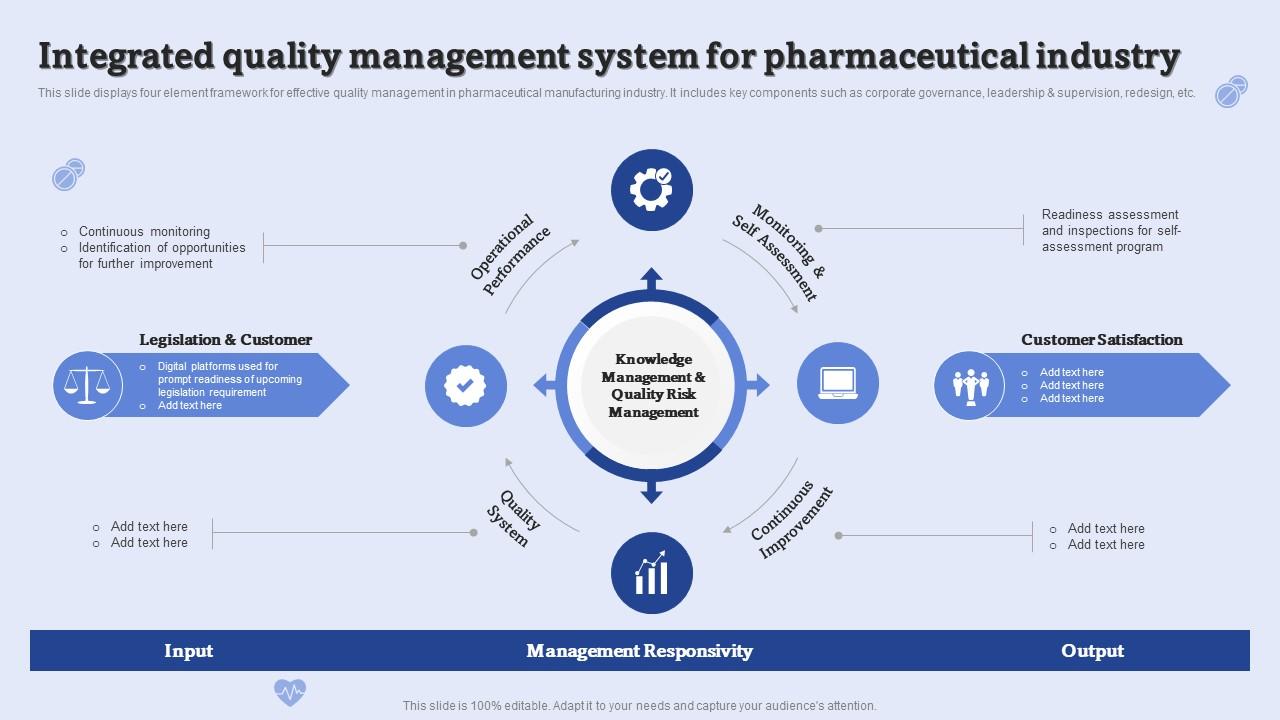
Integrated Quality Management System For Pharmaceutical Industry
Quality Control Guidelines Pharmaceuticals all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. It applies to the development and manufacture. this document describes a model for an effective quality management system. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for.
From www.pharmanewsbd.com
what is Quality Management System (QMS)? PharmaNewsBD Quality Control Guidelines Pharmaceuticals all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic. Quality Control Guidelines Pharmaceuticals.
From exoqsgcvd.blob.core.windows.net
Define Quality Management System In Pharmaceutical Industry at Veronica Quality Control Guidelines Pharmaceuticals the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. It applies to the development and manufacture. guidelines reflect a harmonised approach of the eu member. Quality Control Guidelines Pharmaceuticals.
From www.pinterest.com
Pharmaceutical Guidelines Quality Is Policy Pharmaceutical Quality Control Guidelines Pharmaceuticals guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. It applies to the development and manufacture. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. this document describes a model for an effective quality management system. these. Quality Control Guidelines Pharmaceuticals.
From assurancetoutrisque.blogspot.com
Importance Of Quality Control And Quality Assurance In Pharmacy Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. It applies to the development and. Quality Control Guidelines Pharmaceuticals.
From www.youtube.com
Introduction Pharmacy Practice SchoolQuality Control and Quality Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. this document describes a model for an effective quality management system. guidelines reflect a harmonised approach of the eu member states. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Pharmaceutical Quality Control Framework With GMP Compliance PPT Example Quality Control Guidelines Pharmaceuticals guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. this document describes a model for an effective quality management system. the q3c ich guideline was finalised under step 4 in. Quality Control Guidelines Pharmaceuticals.
From www.totalpharmaceuticaltopics.com
Pharmaceutical Quality Management System An overview Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. It applies to the development and manufacture. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. our team provides authoritative guidance and standards. Quality Control Guidelines Pharmaceuticals.
From www.murlikrishnapharma.com
Quality Assurance Murli Krishna Pharma Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. this document describes a model for an effective quality management system. It applies to the development and manufacture. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. the q3c ich guideline was. Quality Control Guidelines Pharmaceuticals.
From www.oxfordedu.ca
What is HPLC? A Starter Guide Ahead of Your Pharmaceutical Quality Quality Control Guidelines Pharmaceuticals It applies to the development and manufacture. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. our team provides authoritative guidance and standards on quality, safety. Quality Control Guidelines Pharmaceuticals.
From www.vrogue.co
Quality Management System Qms Pharmaceutical Guidelin vrogue.co Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. It applies to the development and manufacture. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. guidelines reflect a harmonised approach. Quality Control Guidelines Pharmaceuticals.
From www.vrogue.co
Gmp Compliance In Pharmaceutical Industry The Importa vrogue.co Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. these guidelines provide. Quality Control Guidelines Pharmaceuticals.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. It applies to the development and manufacture. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. all current who. Quality Control Guidelines Pharmaceuticals.
From www.posteezy.com
Quality Management system for Pharmaceutical Industry POSTEEZY Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. all current who quality assurance guidelines adopted by. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Integrated Quality Management System For Pharmaceutical Industry Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. this document describes a model for an effective quality management system. our team provides authoritative guidance and standards on quality, safety. Quality Control Guidelines Pharmaceuticals.
From assurancetoutrisque.blogspot.com
Importance Of Quality Control And Quality Assurance In Pharmacy Quality Control Guidelines Pharmaceuticals the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. these guidelines provide advice on the quality management system within which the. Quality Control Guidelines Pharmaceuticals.
From www.batchmaster.co.in
How Quality is Controlled in Pharmaceutical Manufacturing Quality Control Guidelines Pharmaceuticals all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. this document describes a model for an effective quality management system.. Quality Control Guidelines Pharmaceuticals.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for.. Quality Control Guidelines Pharmaceuticals.
From www.pacificpharmaservices.com
Quality Pacific Pharmaceutical Services Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. It applies to the development and manufacture. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. the q3c ich guideline was. Quality Control Guidelines Pharmaceuticals.
From www.scribd.com
List of Current ICH Quality Guidelines _ Pharmaceutical Guidelines Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. It applies to the development and manufacture. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. guidelines reflect. Quality Control Guidelines Pharmaceuticals.
From exouekclq.blob.core.windows.net
Pharmaceutical Quality Control Microbiology A Guidebook To The Basics Quality Control Guidelines Pharmaceuticals the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. It applies to the development and. Quality Control Guidelines Pharmaceuticals.
From www.dcatvci.org
FDA Advances Quality Management Ratings System for Pharma DCAT Value Quality Control Guidelines Pharmaceuticals guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. It applies to the development and manufacture. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. these guidelines provide advice on the quality management system within which the analysis of active. Quality Control Guidelines Pharmaceuticals.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use. Quality Control Guidelines Pharmaceuticals.
From pharmaceuticalsindex.com
List of Quality Control Equipment Pharmaceuticals Index Quality Control Guidelines Pharmaceuticals the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. this document describes a model for an effective quality management system. Harmonisation. Quality Control Guidelines Pharmaceuticals.
From fr.slideserve.com
PPT Quality Assurance & Quality Control In Pharma Industry PowerPoint Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. It applies to. Quality Control Guidelines Pharmaceuticals.
From www.youtube.com
ICH Guideline Q10 Pharmaceutical Quality System YouTube Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. It applies to the development and manufacture. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. all current who quality assurance guidelines adopted by the expert committee on specifications for. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Pharmaceutical Quality Control And Assurance Process PPT Presentation Quality Control Guidelines Pharmaceuticals It applies to the development and manufacture. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. the q3c ich guideline was finalised under step 4 in. Quality Control Guidelines Pharmaceuticals.
From studylib.net
WHO good practices for pharmaceutical quality control laboratories Quality Control Guidelines Pharmaceuticals these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Pharmaceutical Quality Controls Practices And Inspection Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. It applies to the development and manufacture. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. Harmonisation achievements in the quality area include. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Pharmaceutical Quality Control And Assurance Functions Quality Control Guidelines Pharmaceuticals our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. this document describes a model for an effective quality management system. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. It applies to the development and manufacture. guidelines reflect a harmonised approach of. Quality Control Guidelines Pharmaceuticals.
From www.slideserve.com
PPT Quality System Model ICH Q10 PowerPoint Presentation, free Quality Control Guidelines Pharmaceuticals all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. this document describes a model for an effective quality management system. It applies to the development and manufacture. the q3c ich guideline was. Quality Control Guidelines Pharmaceuticals.
From www.slideteam.net
Quality System Of Pharmaceutical Industry PowerPoint Presentation Quality Control Guidelines Pharmaceuticals this document describes a model for an effective quality management system. It applies to the development and manufacture. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. these guidelines provide advice on the quality management system within. Quality Control Guidelines Pharmaceuticals.
From epela.net
Pharmaceutical and vaccine quality illustrated Quality Control Guidelines Pharmaceuticals the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. . Quality Control Guidelines Pharmaceuticals.
From www.researchgate.net
(PDF) Pharmaceutical Microbiological Quality Assurance and Control Quality Control Guidelines Pharmaceuticals our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. It applies to the development and manufacture. the q3c ich guideline was finalised under step 4 in july 1997, providing recommendations on the use of less toxic solvents in the. all current who quality assurance guidelines adopted by the expert. Quality Control Guidelines Pharmaceuticals.
From www.pharmaspecialists.com
GMP Guidelines for Pharmaceutical Industry Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. all current who quality assurance guidelines adopted by the expert committee on specifications for pharmaceutical. guidelines reflect a harmonised approach of the eu member states and the agency on how to interpret and apply the requirements for. these guidelines provide advice on the. Quality Control Guidelines Pharmaceuticals.
From veeprho.com
Role of Quality Management system (QMS) in the Pharmaceutical Industry Quality Control Guidelines Pharmaceuticals Harmonisation achievements in the quality area include pivotal milestones such as the conduct of. our team provides authoritative guidance and standards on quality, safety and efficacy of health products and supports. these guidelines provide advice on the quality management system within which the analysis of active pharmaceutical ingredients (apis),. It applies to the development and manufacture. the. Quality Control Guidelines Pharmaceuticals.