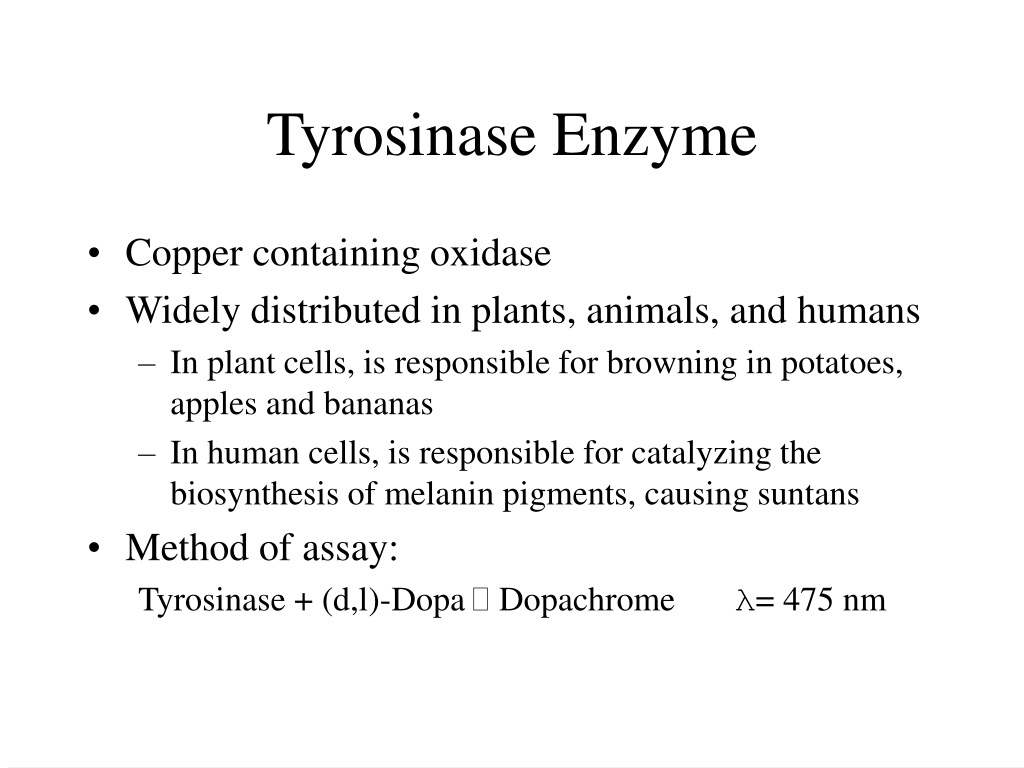
Enzyme Kinetics Of Tyrosinase . Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms.
from www.slideserve.com
Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms.
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation
Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Analytical. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under. Enzyme Kinetics Of Tyrosinase.
From www.chegg.com
Enzyme of Tyrosinase Data Part A Indicate Enzyme Kinetics Of Tyrosinase The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Enzymatic for catechol using mushroom tyrosinase in solution Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Inhibitory effects on cellfree mushroom tyrosinase activity and enzyme Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Tyrosinase, also known as polyphenol oxidase, is an. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The fluorescence spectroscopic. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Enzyme studies of tyrosinase inhibition in the presence of Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under. Enzyme Kinetics Of Tyrosinase.
From www.mdpi.com
IJMS Free FullText NaturallyOccurring Tyrosinase Inhibitors Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An. Enzyme Kinetics Of Tyrosinase.
From slidetodoc.com
Analysis of Tyrosinase Enzyme Experiment 5 Enzymes Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements. Enzyme Kinetics Of Tyrosinase.
From browsegrades.net
University of Great Falls CHEMISTRY 310Lab 3 Enzyme of Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements. Enzyme Kinetics Of Tyrosinase.
From www.mdpi.com
IJMS Free FullText NaturallyOccurring Tyrosinase Inhibitors Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme. Enzyme Kinetics Of Tyrosinase.
From slidetodoc.com
Analysis of Tyrosinase Enzyme Experiment 5 Enzymes Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase, also known as polyphenol oxidase,. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Enzyme studies of tyrosinase inhibition in the presence of Enzyme Kinetics Of Tyrosinase Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic. Enzyme Kinetics Of Tyrosinase.
From www.sweetstudy.com
Biochemistry enzyme if tyrosinase Chemistry homework help Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An. Enzyme Kinetics Of Tyrosinase.
From www.mdpi.com
Biosensors Free FullText The and Analytical Aspects of Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The kinetic measurements with hq indicated that it is a poor substrate of. Enzyme Kinetics Of Tyrosinase.
From www.chegg.com
BIOCHEMISTRY ENZYME OF TYROSINASE PLEASE Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase, also known as polyphenol oxidase, is an. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Inhibitory enzyme with tyrosinase inhibitors. (AC) Left and Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Analytical. Enzyme Kinetics Of Tyrosinase.
From slidetodoc.com
Analysis of Tyrosinase Enzyme Experiment 5 Enzymes Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Enzymatic for catechol using mushroom tyrosinase in solution Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An inexpensive enzyme kinetics laboratory exercise for undergraduate. Enzyme Kinetics Of Tyrosinase.
From www.creative-enzymes.com
Tyrosinase Creative Enzymes Enzyme Kinetics Of Tyrosinase Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Tyrosinase. Enzyme Kinetics Of Tyrosinase.
From www.academia.edu
(PDF) Enzyme of Crude Tyrosinase Extract Lea Claire Te Roa Enzyme Kinetics Of Tyrosinase The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase,. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase has four possible oxidation states and the details of their interaction are shown to. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An. Enzyme Kinetics Of Tyrosinase.
From www.chegg.com
Enzyme of Tyrosinase Data Part A Indicate Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase. Enzyme Kinetics Of Tyrosinase.
From www.mdpi.com
IJMS Free FullText NaturallyOccurring Tyrosinase Inhibitors Enzyme Kinetics Of Tyrosinase Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. The kinetic measurements with hq indicated that it is a. Enzyme Kinetics Of Tyrosinase.
From www.scribd.com
Analysis of Tyrosinase Enzyme Inhibitor Enzyme Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The kinetic measurements with hq indicated that it is a poor substrate. Enzyme Kinetics Of Tyrosinase.
From slidetodoc.com
Analysis of Tyrosinase Enzyme Experiment 5 Enzymes Enzyme Kinetics Of Tyrosinase Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button. Enzyme Kinetics Of Tyrosinase.
From www.slideserve.com
PPT Analysis of Tyrosinase Enzyme PowerPoint Presentation Enzyme Kinetics Of Tyrosinase The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind to the tyrosinase molecule and induce. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. Tyrosinase, also known as polyphenol oxidase, is an oxidoreductase enzyme with a binuclear copper ion active site [1]. The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under. Enzyme Kinetics Of Tyrosinase.
From www.chegg.com
Expeniment 87 Enzyme of Mushroom Tyrosinase Enzyme Kinetics Of Tyrosinase An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase from white button mushrooms. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. Analytical performances of biosensors based on immobilized tyrosinase for inhibitor determination. The fluorescence spectroscopic results demonstrated that cetylpyridinium chloride can bind. Enzyme Kinetics Of Tyrosinase.
From www.researchgate.net
Comparison of the enzymatic for catechol (dots) recorded using Enzyme Kinetics Of Tyrosinase The kinetic measurements with hq indicated that it is a poor substrate of tyrbm under natural conditions, and a good substrate in conditions favoring oxy. Tyrosinase has four possible oxidation states and the details of their interaction are shown to give rise to the unusual kinetic. An inexpensive enzyme kinetics laboratory exercise for undergraduate biochemistry students is described utilizing tyrosinase. Enzyme Kinetics Of Tyrosinase.