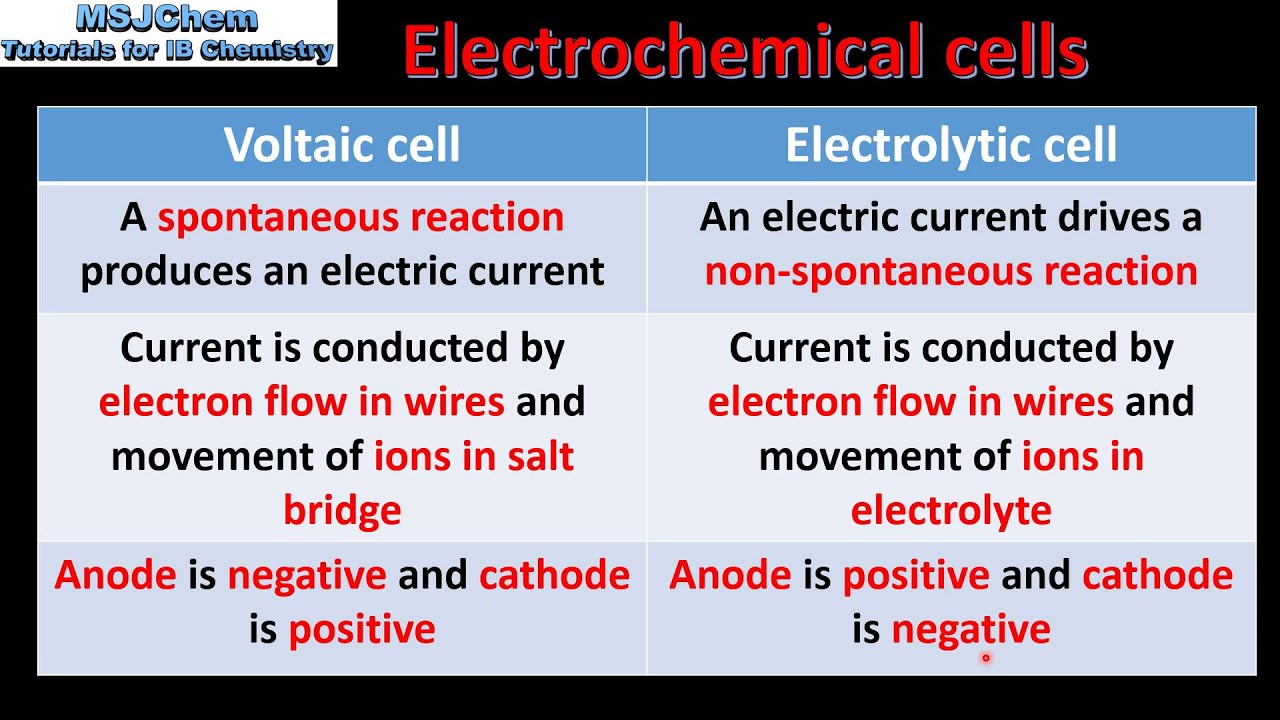
What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell . In an electrochemical cell, half cells are placed in different containers and connected via a. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell converts chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: However, there are also striking differences between the two cells. A galvanic cell (left) transforms the energy. The main differences are outlined below:
from www.youtube.com
An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: A galvanic cell (left) transforms the energy. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. However, there are also striking differences between the two cells. The main differences are outlined below: In an electrochemical cell, half cells are placed in different containers and connected via a. An electrochemical cell converts chemical energy into electrical. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for.
9.2 Comparison of electrochemical cells (SL) YouTube
What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. An electrochemical cell converts chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: The main differences are outlined below: However, there are also striking differences between the two cells. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. In an electrochemical cell, half cells are placed in different containers and connected via a. A galvanic cell (left) transforms the energy.
From www.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. A galvanic cell (left) transforms the energy. An electrochemical cell converts chemical energy into. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From mavink.com
Diagram Of Electrochemical Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. However, there are also striking differences between the two cells. An electrochemical cell converts chemical energy into electrical. In an electrochemical cell, half. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. An electrochemical cell converts chemical energy into electrical. A galvanic cell (left) transforms the energy.. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: A galvanic cell (left) transforms the energy. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell converts chemical energy into electrical. However, there are also striking. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell converts chemical energy into electrical. The main differences are outlined below: The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. A galvanic cell (left) transforms the energy. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. However, there are also striking differences between the two cells. A galvanic cell (left) transforms the energy. An electrochemical cell converts chemical energy into electrical. The main difference between an electrochemical cell and an electrolytic cell lies in their. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). A galvanic cell (left) transforms the energy. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. However, there are. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.askdifference.com
Electrochemical Cell vs. Electrolytic Cell — What’s the Difference? What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. However, there are also striking differences between the two cells. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell splits the oxidant and reductant in a manner. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. The main differences are outlined below: The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: When an electric current. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.chemca.in
Types of Electrochemical Cells Difference between galvanic cell and What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell converts chemical energy into electrical. A galvanic cell (left) transforms the energy. The most significant difference between an electrolytic cell and. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell converts chemical energy into electrical. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell splits the oxidant and. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). The main difference between an electrochemical cell and an electrolytic. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell converts chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). However, there are also striking differences between the two cells.. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. A galvanic cell (left) transforms the energy. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). The main differences. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: However, there are also. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From chem2u.blogspot.com
chem2U Elelctrolytic Cell and Chemical Cells What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. In an electrochemical cell, half cells are placed in different containers and connected via a. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell splits the oxidant and reductant in a. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From stoplearn.com
Electrochemical Cells 2023 What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). A galvanic cell (left) transforms the energy. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: An electrochemical cell converts chemical energy into electrical. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: In an electrochemical cell, half cells are placed in different containers and connected via a. The most significant difference between an electrolytic cell and an electrochemical cell is that an. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From knowdifferences.com
Difference between Electrochemical Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell converts chemical energy into electrical. The main differences are outlined below: When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. An. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From thechemistrynotes.com
Electrolytic Cell Definition, Principle, Components, Applications What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. However, there are also striking differences between the two cells. An electrochemical cell converts chemical energy into electrical. A galvanic cell (left) transforms the energy. Electrochemical cells and electrolytic cells are both fundamental in the field. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). In an electrochemical cell, half cells are placed in different containers and connected via a.. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: However, there are also striking differences between the two cells. In an electrochemical cell, half cells are placed in different containers and connected via a. A galvanic cell (left) transforms the energy. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From askfilo.com
These are of two types Electrochemical cell and Electrolytic cell. Sr. N.. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell converts chemical energy into electrical. In an electrochemical cell, half cells are. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell A galvanic cell (left) transforms the energy. However, there are also striking differences between the two cells. In an electrochemical cell, half cells are placed in different containers and connected via a. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. The main differences are outlined below: An. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. An electrochemical cell converts chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.es
What are the differences between Galvanic cell and Electrolytic cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. In an electrochemical cell, half cells are placed in different containers and connected. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell converts chemical. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.artofit.org
Electrochemical cell Artofit What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. A galvanic cell (left) transforms the energy. In an electrochemical cell, half cells are placed in different containers and connected via a. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.com
Difference Between Electrochemical Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: An electrochemical cell converts chemical energy into electrical. Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation at the anode and reduction at. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell converts electrical. The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: A. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell converts chemical energy into electrical. A galvanic cell (left) transforms the energy. The main differences are outlined below: Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. The most. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell The main difference between an electrochemical cell and an electrolytic cell lies in their operational direction: An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets oxidized). Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected via a. The main differences are outlined below: Electrochemical cells and electrolytic cells are both fundamental in the field of chemistry, but they operate on different principles and for. When an electric current is passed through the cell, ions migrate towards the electrodes, where they undergo oxidation. What Is The Main Difference Between Electrochemical Cell And Electrolytic Cell.