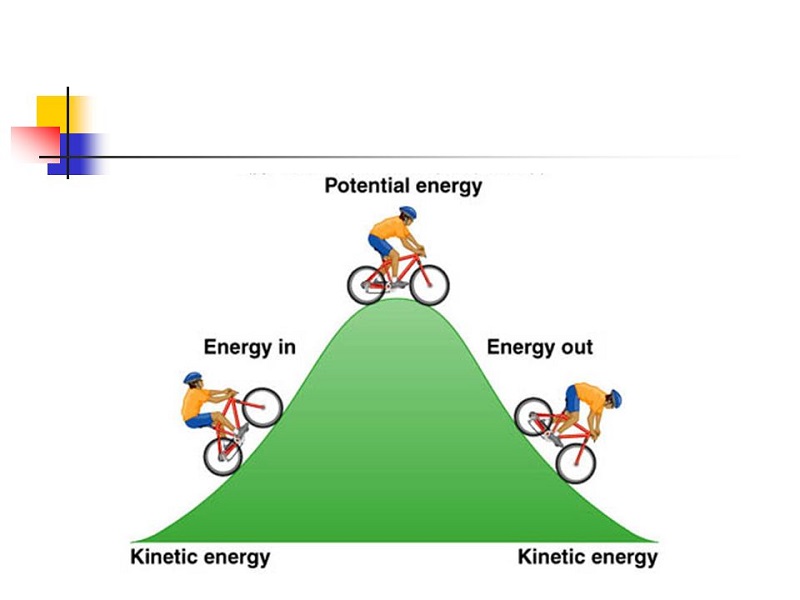
Threshold Energy In Chemistry In Hindi . जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. It is the minimum amount of energy which the reactant molecules must possess for the. Step by step video, text & image solution for threshold energy is equal to. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Using the concept of activation energy, explain the role of a cataylst on the rate of. Define the terms threshold energy and activation energy. The average internal energy is the energy that your reactant molecule has internally. A) derive an integrated rate equation for rate constant of a first order reaction. The threshold energy is the energy that these.
from vigyanam.com
देहली अभिक्रिया ऊर्जा (threshold reaction energy) : The threshold energy is the energy that these. The average internal energy is the energy that your reactant molecule has internally. Define the terms threshold energy and activation energy. A) derive an integrated rate equation for rate constant of a first order reaction. Step by step video, text & image solution for threshold energy is equal to. Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. It is the minimum amount of energy which the reactant molecules must possess for the.
All About Energy In Hindi ऊर्जा की पूरी सटीक जानकारी और उसके प्रकार
Threshold Energy In Chemistry In Hindi Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. It is the minimum amount of energy which the reactant molecules must possess for the. Step by step video, text & image solution for threshold energy is equal to. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. A) derive an integrated rate equation for rate constant of a first order reaction. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. Using the concept of activation energy, explain the role of a cataylst on the rate of. Define the terms threshold energy and activation energy. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : The average internal energy is the energy that your reactant molecule has internally. The threshold energy is the energy that these.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Threshold Energy In Chemistry In Hindi जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. The average internal energy is the energy that your reactant molecule has internally. The threshold energy is the energy that these.. Threshold Energy In Chemistry In Hindi.
From byjus.com
What is the effect of catalyst on threshold energy? Threshold Energy In Chemistry In Hindi The threshold energy is the energy that these. Step by step video, text & image solution for threshold energy is equal to. It is the minimum amount of energy which the reactant molecules must possess for the. Using the concept of activation energy, explain the role of a cataylst on the rate of. देहली अभिक्रिया ऊर्जा (threshold reaction energy) :. Threshold Energy In Chemistry In Hindi.
From freakylearn.com
"energy" Meaning in Hindi FreakyLearn Threshold Energy In Chemistry In Hindi It is the minimum amount of energy which the reactant molecules must possess for the. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. The average internal energy is the energy that your reactant molecule has internally. Threshold energy is the minimum energy that all colliding molecules. Threshold Energy In Chemistry In Hindi.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy In Chemistry In Hindi देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Define the terms threshold energy and activation energy. The average internal energy is the energy that your reactant molecule has internally. Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make. Threshold Energy In Chemistry In Hindi.
From vigyanam.com
All About Energy In Hindi ऊर्जा की पूरी सटीक जानकारी और उसके प्रकार Threshold Energy In Chemistry In Hindi जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. Step by step video, text & image solution for threshold energy is equal to. The average internal energy is the energy that your reactant molecule has internally. Using the concept of activation energy, explain the role of a. Threshold Energy In Chemistry In Hindi.
From www.eclubstudy.com
उर्जा के स्रोत क्या है Sources of Energy in Hindi Threshold Energy In Chemistry In Hindi Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. It is the minimum amount of energy which the reactant molecules must possess for the. The average internal energy is the energy that your reactant molecule has internally. Step by step video, text & image solution for threshold energy is equal to. The. Threshold Energy In Chemistry In Hindi.
From www.toppr.com
The energy profile for the reaction CO(g) + NO2(g) CO2(g) + NO(g) is Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. Define the terms threshold energy and activation energy. The threshold energy is the energy that these. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. The average internal energy is the. Threshold Energy In Chemistry In Hindi.
From www.meritnation.com
The binding energy of electron in a metal is 250 kJ/mol The threshold Threshold Energy In Chemistry In Hindi जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. Step by step video, text & image solution for threshold energy is equal to. It is the minimum amount of energy which the reactant molecules must possess for the. Define the terms threshold energy and activation energy. Threshold. Threshold Energy In Chemistry In Hindi.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy In Chemistry In Hindi The average internal energy is the energy that your reactant molecule has internally. A) derive an integrated rate equation for rate constant of a first order reaction. Define the terms threshold energy and activation energy. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. Step by step video, text & image solution. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Calculate threshold frequency Dual nature of light Physics Khan Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. A) derive an integrated rate equation for rate constant of a first order reaction. Define the terms threshold energy and activation energy. जब कोई ऊर्जावान कण किसी. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical Threshold Energy In Chemistry In Hindi The average internal energy is the energy that your reactant molecule has internally. Step by step video, text & image solution for threshold energy is equal to. It is the minimum amount of energy which the reactant molecules must possess for the. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. Using. Threshold Energy In Chemistry In Hindi.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy In Chemistry In Hindi Step by step video, text & image solution for threshold energy is equal to. The threshold energy is the energy that these. Define the terms threshold energy and activation energy. Using the concept of activation energy, explain the role of a cataylst on the rate of. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : It is the minimum amount of energy. Threshold Energy In Chemistry In Hindi.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy In Chemistry In Hindi Define the terms threshold energy and activation energy. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. It is the minimum amount of energy which the reactant molecules must possess for the. Step by step video, text & image solution for threshold energy is equal to. A) derive an integrated rate equation. Threshold Energy In Chemistry In Hindi.
From byjus.com
select the incorrect statement (1) The minimum amount of energy Threshold Energy In Chemistry In Hindi A) derive an integrated rate equation for rate constant of a first order reaction. It is the minimum amount of energy which the reactant molecules must possess for the. Using the concept of activation energy, explain the role of a cataylst on the rate of. Define the terms threshold energy and activation energy. Threshold energy is the minimum energy that. Threshold Energy In Chemistry In Hindi.
From kunduz.com
[ANSWERED] What is the threshold energy of reaction RP in represented Threshold Energy In Chemistry In Hindi Step by step video, text & image solution for threshold energy is equal to. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. It is the minimum amount of energy which the reactant molecules must possess for the. Define the terms threshold energy and activation energy. Using. Threshold Energy In Chemistry In Hindi.
From scoop.eduncle.com
What is the formula to find q value and threshold energy of a nuclear Threshold Energy In Chemistry In Hindi It is the minimum amount of energy which the reactant molecules must possess for the. A) derive an integrated rate equation for rate constant of a first order reaction. Define the terms threshold energy and activation energy. Using the concept of activation energy, explain the role of a cataylst on the rate of. Step by step video, text & image. Threshold Energy In Chemistry In Hindi.
From hxebhtpaz.blob.core.windows.net
Threshold Energy In Chemistry at Merle Buchanan blog Threshold Energy In Chemistry In Hindi It is the minimum amount of energy which the reactant molecules must possess for the. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. Step by step video, text & image solution for threshold energy is equal to. Using the concept of activation energy, explain the role of a cataylst on the. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Threshold Energy of Nuclear Reaction Threshold Energy derivation In Threshold Energy In Chemistry In Hindi Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. The threshold energy is the energy that these. Step by step video, text & image solution for threshold energy is equal to. The average internal energy is the energy that your reactant molecule has internally. Using the concept of activation energy, explain the. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Concept of Activation Energy, Threshold Energy Chemistry(12th Threshold Energy In Chemistry In Hindi Step by step video, text & image solution for threshold energy is equal to. The threshold energy is the energy that these. Using the concept of activation energy, explain the role of a cataylst on the rate of. A) derive an integrated rate equation for rate constant of a first order reaction. Threshold energy is the minimum energy that all. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. Define the terms threshold energy and activation energy. Step by step video, text & image solution for threshold energy is equal to. A) derive an integrated rate equation for rate constant of a first order reaction. It is the minimum amount of energy which. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Nuclear Physics (Qvalue, Threshold Energy) YouTube Threshold Energy In Chemistry In Hindi Define the terms threshold energy and activation energy. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : The average internal energy is the. Threshold Energy In Chemistry In Hindi.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy In Chemistry In Hindi देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Using the concept of activation energy, explain the role of a cataylst on the rate of. The average internal energy is the energy that your reactant molecule has internally. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. It is the minimum amount of energy. Threshold Energy In Chemistry In Hindi.
From www.numerade.com
SOLVEDDetermine the Q value and the threshold energy for 8^16 O+ 0^1 Threshold Energy In Chemistry In Hindi The average internal energy is the energy that your reactant molecule has internally. A) derive an integrated rate equation for rate constant of a first order reaction. Define the terms threshold energy and activation energy. Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding. Threshold Energy In Chemistry In Hindi.
From www.doubtnut.com
In a reaction, the threshold energy is equal to Threshold Energy In Chemistry In Hindi जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. Step by step video, text & image solution for threshold energy is equal to. A) derive an integrated rate equation for rate constant of a first order reaction. It is the minimum amount of energy which the reactant. Threshold Energy In Chemistry In Hindi.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy In Chemistry In Hindi It is the minimum amount of energy which the reactant molecules must possess for the. Define the terms threshold energy and activation energy. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. A) derive an integrated rate equation for rate constant of a first order. Threshold Energy In Chemistry In Hindi.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : The threshold energy is the energy that these. The average internal energy is the energy that your reactant molecule has internally. Step by step video, text & image solution for threshold energy is equal to. Threshold energy. Threshold Energy In Chemistry In Hindi.
From howtova.blogspot.com
How To Calculate Threshold Frequency HOWTOVA Threshold Energy In Chemistry In Hindi Define the terms threshold energy and activation energy. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. The threshold energy is the energy that these. Using the concept of activation energy, explain the role of a cataylst on the rate of. A) derive an integrated rate equation. Threshold Energy In Chemistry In Hindi.
From www.doubtnut.com
The Threshold energy is given as E(0) and radiation of energy E Threshold Energy In Chemistry In Hindi देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Step by step video, text & image solution for threshold energy is equal to. A) derive an integrated rate equation for rate constant of a first order reaction. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर कण से. Threshold Energy In Chemistry In Hindi.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy In Chemistry In Hindi It is the minimum amount of energy which the reactant molecules must possess for the. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में. Threshold Energy In Chemistry In Hindi.
From ethiopialearning.com
Ethiopia Learning Chemistry grade 11 page 71 in English Threshold Energy In Chemistry In Hindi A) derive an integrated rate equation for rate constant of a first order reaction. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर. Threshold Energy In Chemistry In Hindi.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy In Chemistry In Hindi Define the terms threshold energy and activation energy. Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : The threshold energy is the energy that these. Step by step video,. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
13.18 How to calculate the energy released in a nuclear reaction YouTube Threshold Energy In Chemistry In Hindi The average internal energy is the energy that your reactant molecule has internally. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. Using the concept of activation energy, explain the role of a cataylst on the rate of. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना. Threshold Energy In Chemistry In Hindi.
From www.youtube.com
Sources of energy class 10 in hindi Lecture 01 YouTube Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. Step by step video, text & image solution for threshold energy is equal to. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : A) derive an integrated rate equation for rate constant of a first order reaction. Threshold energy is the minimum energy that all colliding. Threshold Energy In Chemistry In Hindi.
From www.meritnation.com
The threshold frequency v0 for a metal is 5 0 *10 ^14s what will be the Threshold Energy In Chemistry In Hindi Using the concept of activation energy, explain the role of a cataylst on the rate of. Threshold energy is the minimum energy that all colliding molecules must possess in order to make the. जब कोई ऊर्जावान कण किसी स्थिर कण से टक्कर करता है तो यह सम्भावना हो सकती है कि इस अभिक्रिया में कई. The average internal energy is. Threshold Energy In Chemistry In Hindi.
From www.toppr.com
The threshold energy of a metal is 10 sV. Identify the incorrect option Threshold Energy In Chemistry In Hindi A) derive an integrated rate equation for rate constant of a first order reaction. देहली अभिक्रिया ऊर्जा (threshold reaction energy) : Using the concept of activation energy, explain the role of a cataylst on the rate of. It is the minimum amount of energy which the reactant molecules must possess for the. The threshold energy is the energy that these.. Threshold Energy In Chemistry In Hindi.