Fda Animal Testing Requirements Drugs . Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. New medicines need not be tested in animals to receive u.s. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs.
from www.fdareview.org
Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. New medicines need not be tested in animals to receive u.s.
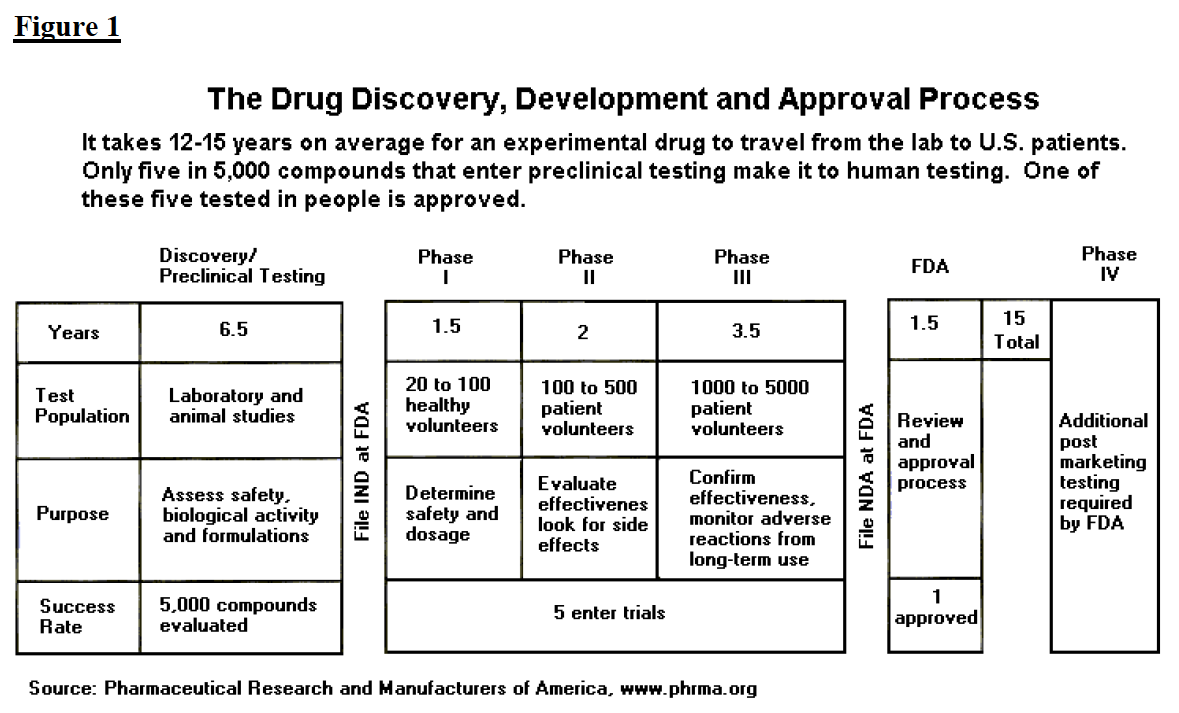
The Drug Development and Approval Process
Fda Animal Testing Requirements Drugs This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. New medicines need not be tested in animals to receive u.s. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs.
From vegnews.com
13 Bipartisan Lawmakers Demand FDA Set Guidelines for AnimalFree Drug Fda Animal Testing Requirements Drugs As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. New medicines need not be tested in animals to receive u.s. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. Law has eliminated the requirement that. Fda Animal Testing Requirements Drugs.
From www.techtimes.com
New FDA Law Ends Animal Testing Requirement for Drugs, Advances Humane Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. New medicines need not be tested in animals to receive u.s. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. As it now stands, the fda. Fda Animal Testing Requirements Drugs.
From animalia-life.club
Fda Drug Labeling Requirements Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december. Fda Animal Testing Requirements Drugs.
From www.slideserve.com
PPT Animal Experimentation PowerPoint Presentation, free download Fda Animal Testing Requirements Drugs The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Law has eliminated the requirement that drugs in development must undergo. Fda Animal Testing Requirements Drugs.
From plantbasednews.org
Pharmaceutical Animal Tests Are No Longer Required In The USA Fda Animal Testing Requirements Drugs New medicines need not be tested in animals to receive u.s. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. Animal testing is no. Fda Animal Testing Requirements Drugs.
From www.fdli.org
Animal Drug Compounding FDA’s New Guidance and Impacts on Industry Fda Animal Testing Requirements Drugs New medicines need not be tested in animals to receive u.s. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. Food and drug. Fda Animal Testing Requirements Drugs.
From secure.everyaction.com
Petition to FDA Eliminate unreliable FDA animal testing requirements Fda Animal Testing Requirements Drugs Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. This meant that when the fda found time to test a pharmaceutical or other compound being. Fda Animal Testing Requirements Drugs.
From www.youtube.com
Why We Should Use Animals For Testing Drugs, Food, Chemicals, and Fda Animal Testing Requirements Drugs Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. But the safety and efficacy of drug candidates must still be determined before they are administered. Fda Animal Testing Requirements Drugs.
From kids.frontiersin.org
Replacing Animal Testing How and When? · Frontiers for Young Minds Fda Animal Testing Requirements Drugs This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. But the safety and efficacy of drug candidates must still be determined before they. Fda Animal Testing Requirements Drugs.
From rzrnews.com
RZR News The New Way To News Fda Animal Testing Requirements Drugs This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. New medicines need not be tested in animals to receive u.s. Food and. Fda Animal Testing Requirements Drugs.
From suchscience.net
Understanding FDA Animal Testing Fda Animal Testing Requirements Drugs But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. This meant that when the fda found time to test a pharmaceutical or other compound being. Fda Animal Testing Requirements Drugs.
From www.alston.com
FDA/Food, Drug & Device Advisory FDA Proposes to Overhaul Labeling Fda Animal Testing Requirements Drugs Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. New medicines need not be tested in animals to receive u.s. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. Food and. Fda Animal Testing Requirements Drugs.
From www.worldhealthcarereport.com
Congress Approves Landmark Measure to Reduce Animal Testing World Fda Animal Testing Requirements Drugs Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. Animal testing is no longer required for new drugs, marking a triumph in. Fda Animal Testing Requirements Drugs.
From www.zeclinics.com
FDA no longer requires animal testing to test drugs in humans ZeClinics Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of. Fda Animal Testing Requirements Drugs.
From transcurebioservices.com
The FDA removes animal testing requirement for drug candidates Fda Animal Testing Requirements Drugs Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. New medicines need not be tested in animals to receive u.s. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. This meant that when the. Fda Animal Testing Requirements Drugs.
From www.treehugger.com
Everything You Need to Know About Animal Testing for Cosmetics Fda Animal Testing Requirements Drugs The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. Law has eliminated the requirement that drugs in development must undergo testing in. Fda Animal Testing Requirements Drugs.
From animal.research.uiowa.edu
Substance Administration Use of Drugs and Chemicals in Laboratory Fda Animal Testing Requirements Drugs This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. As it now stands, the fda requires toxicity tests on one rodent species (eg,. Fda Animal Testing Requirements Drugs.
From www.detroitcatholic.com
Animal welfare groups hail FDA decision to lift animal testing Fda Animal Testing Requirements Drugs The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. Food and drug administration (fda) approval, according to legislation signed by. Fda Animal Testing Requirements Drugs.
From suchscience.net
Understanding FDA Animal Testing Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat). Fda Animal Testing Requirements Drugs.
From patsnap01.blogspot.com
The Ethical Evolution of Animal Testing FDA's New Rules Fda Animal Testing Requirements Drugs As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. New medicines need not be tested in animals to receive u.s. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Food and drug. Fda Animal Testing Requirements Drugs.
From www.regdesk.co
FDA on BCI Devices Animal Testing RegDesk Fda Animal Testing Requirements Drugs Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent. Fda Animal Testing Requirements Drugs.
From lestwinsonline.com
Top 141 + Drugs developed through animal testing Fda Animal Testing Requirements Drugs As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. The change—long sought by animal welfare organizations—could signal a major shift away from animal use. Fda Animal Testing Requirements Drugs.
From cytosolve.com
FDA aims to reduce animal testing in drug research CytoSolve Fda Animal Testing Requirements Drugs New medicines need not be tested in animals to receive u.s. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. This meant. Fda Animal Testing Requirements Drugs.
From www.drugs.com
FDA Drug Approval Process Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late. Fda Animal Testing Requirements Drugs.
From solutionpharmacy.in
Animal Toxicity Testing Solution Parmacy Fda Animal Testing Requirements Drugs Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. New medicines need not be tested in animals to receive u.s. This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Drugs evaluated for efficacy under the animal rule. Fda Animal Testing Requirements Drugs.
From www.fdareview.org
The Drug Development and Approval Process Fda Animal Testing Requirements Drugs The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. But the safety and efficacy of drug candidates must still be determined before they are. Fda Animal Testing Requirements Drugs.
From keplarllp.com
🎉 Drug testing on animals pros and cons. The Pros and Cons of Drug Fda Animal Testing Requirements Drugs Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing. Fda Animal Testing Requirements Drugs.
From xborderusa.com
US Food and Drug Administration (FDA) Registration What You Need to Fda Animal Testing Requirements Drugs The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. But the safety and efficacy of drug candidates must still be determined before they are administered to humans in phase 1, 2 or 3 clinical trials. As it now stands, the fda requires toxicity tests on one. Fda Animal Testing Requirements Drugs.
From www.science.org
FDA no longer needs to require animal tests before human drug trials Fda Animal Testing Requirements Drugs This meant that when the fda found time to test a pharmaceutical or other compound being sold to the public, safety testing in. Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal. Fda Animal Testing Requirements Drugs.
From www.allevi3d.com
FDA Modernization Act highlights advances in animalfree preclinical Fda Animal Testing Requirements Drugs Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. The change—long sought by animal welfare organizations—could signal a major shift away from animal use. Fda Animal Testing Requirements Drugs.
From modernizetesting.org
FDA’s Animal Testing Requirement Harms People and Animals and Wastes Fda Animal Testing Requirements Drugs Animal testing is no longer required for new drugs, marking a triumph in scientific research and technology and animal rights. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. New medicines need not be tested in animals to receive u.s. As it now stands, the. Fda Animal Testing Requirements Drugs.
From www.slideshare.net
FDA Animals Testing Fda Animal Testing Requirements Drugs As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80 years of drug safety. But the safety and efficacy of drug candidates must still be determined before they. Fda Animal Testing Requirements Drugs.
From modernizetesting.org
Paul, Booker, Braun, Lujan, and Kennedy Introduce FDA Modernization Act Fda Animal Testing Requirements Drugs Food and drug administration (fda) approval, according to legislation signed by president joe biden in late december 2022. As it now stands, the fda requires toxicity tests on one rodent species (eg, mouse or rat) and one nonrodent species (eg, nonhuman. The change—long sought by animal welfare organizations—could signal a major shift away from animal use after more than 80. Fda Animal Testing Requirements Drugs.
From www.caareusa.org
Help save animals from cruel drug tests Citizens for Alternatives to Fda Animal Testing Requirements Drugs New medicines need not be tested in animals to receive u.s. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. As it now. Fda Animal Testing Requirements Drugs.
From www.yumpu.com
FDA The New Animal Drug Approval Process Fda Animal Testing Requirements Drugs Law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials. Drugs evaluated for efficacy under the animal rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs. This meant that when the fda found time to test a pharmaceutical or other. Fda Animal Testing Requirements Drugs.