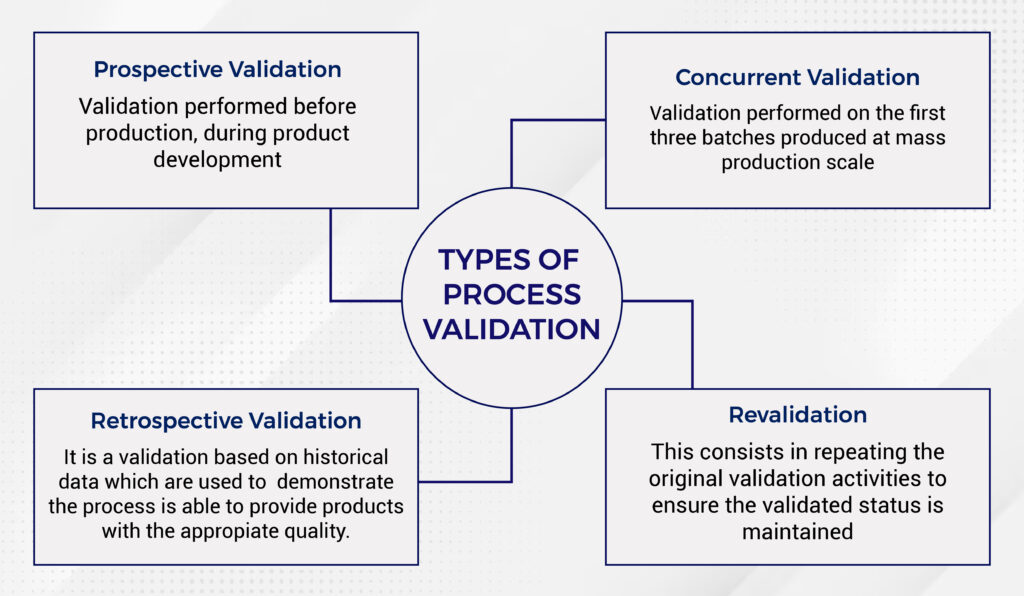
Medical Device Process Validation Example . Process verification and process validation are two important—and commonly misunderstood—activities in the development. Understanding iq, oq, and pq for medical device manufacturing processes. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. As part of design and development validation, the organization shall perform clinical evaluations or performance. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. The goal of process validation is to produce a stable. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical.
from operonstrategist.com
• performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Understanding iq, oq, and pq for medical device manufacturing processes. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Process verification and process validation are two important—and commonly misunderstood—activities in the development. The goal of process validation is to produce a stable. As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be.
Guide to Medical Device Process Validation in Manufacturing Operon
Medical Device Process Validation Example Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. As part of design and development validation, the organization shall perform clinical evaluations or performance. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation involves a series of activities taking place over the lifecycle of the product and process. Understanding iq, oq, and pq for medical device manufacturing processes. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. The goal of process validation is to produce a stable. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be.
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Medical Device Process Validation Example Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Process verification and process validation are two important—and commonly misunderstood—activities in the development. As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples. Medical Device Process Validation Example.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Process Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation serves as a proactive measure, identifying potential issues from failure. Medical Device Process Validation Example.
From www.slideshare.net
Process Validation for Medical Device Compliance Medical Device Process Validation Example The goal of process validation is to produce a stable. Understanding iq, oq, and pq for medical device manufacturing processes. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the. Medical Device Process Validation Example.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is to produce a stable. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process verification and process validation are two important—and commonly misunderstood—activities in the development.. Medical Device Process Validation Example.
From rs-ness.com
Medical Device Packaging Validation Guideline RS NESS Medical Device Process Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process validation involves a series of activities taking. Medical Device Process Validation Example.
From www.slideshare.net
Effective medical device validation introduction manual advance Medical Device Process Validation Example Understanding iq, oq, and pq for medical device manufacturing processes. As part of design and development validation, the organization shall perform clinical evaluations or performance. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. According to guidance published by the ghtf (now the imdrf), the following are common examples of. Medical Device Process Validation Example.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Process Validation Example Understanding iq, oq, and pq for medical device manufacturing processes. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process verification and process validation are two important—and commonly misunderstood—activities in the development.. Medical Device Process Validation Example.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Medical Device Process Validation Example Understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation is to produce a stable. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. As part of design and development validation, the organization shall perform clinical evaluations or performance. Process validation serves as a. Medical Device Process Validation Example.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Process Validation Example The goal of process validation is to produce a stable. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Understanding iq, oq, and pq for medical device manufacturing processes. According to guidance published. Medical Device Process Validation Example.
From www.slideteam.net
Development Plan Of New Clinical Device With Verification And Medical Device Process Validation Example Understanding iq, oq, and pq for medical device manufacturing processes. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Process validation involves a series of activities taking place over the lifecycle of the product and process. According to guidance. Medical Device Process Validation Example.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Process Validation Example Process verification and process validation are two important—and commonly misunderstood—activities in the development. Understanding iq, oq, and pq for medical device manufacturing processes. As part of design and development validation, the organization shall perform clinical evaluations or performance. The goal of process validation is to produce a stable. • performing process validation ensure that the process output is predictable and. Medical Device Process Validation Example.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is to produce a stable. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Process verification and process validation are two important—and commonly misunderstood—activities in the development.. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device ValidationPresentationEZE Medical Device Process Validation Example Process validation involves a series of activities taking place over the lifecycle of the product and process. Understanding iq, oq, and pq for medical device manufacturing processes. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process validation serves as a proactive measure, identifying potential issues from failure in production. Medical Device Process Validation Example.
From www.cognidox.com
Design verification & design validation for medical device developers Medical Device Process Validation Example The goal of process validation is to produce a stable. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Understanding iq, oq, and pq for medical device manufacturing processes. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process. Medical Device Process Validation Example.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. As part of design and development validation, the organization shall perform clinical evaluations or performance. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process verification and process validation are. Medical Device Process Validation Example.
From data1.skinnyms.com
Medical Device Verification And Validation Plan Template Medical Device Process Validation Example The goal of process validation is to produce a stable. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process verification and process validation are two important—and commonly misunderstood—activities in the development.. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Process Validation Example Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation is to produce a stable. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. According to guidance published. Medical Device Process Validation Example.
From www.slideteam.net
Medical Device Process Validation Flowchart Medical Device Process Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is. Medical Device Process Validation Example.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Process Validation Example • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation involves a series of activities taking place over the lifecycle of the product and process. Understanding iq, oq, and pq for medical device manufacturing processes. Process. Medical Device Process Validation Example.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Process Validation Example Process validation involves a series of activities taking place over the lifecycle of the product and process. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. The goal of process validation is to produce a stable. Process validation serves as a proactive measure, identifying potential issues from failure in production. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Understanding iq, oq, and pq for medical device manufacturing processes. As part of design and development validation, the organization shall perform clinical evaluations or performance. Process validation serves as a proactive measure, identifying potential issues from failure in production before. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Medical Device Process Validation Example Understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation is to produce a stable. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation involves a series of activities taking place over the lifecycle of the product and process. • performing process validation ensure that the process output is. Medical Device Process Validation Example.
From swarali-bhakre.medium.com
Process Validation for medical devices by IZiel Healthcare Medium Medical Device Process Validation Example Process validation involves a series of activities taking place over the lifecycle of the product and process. As part of design and development validation, the organization shall perform clinical evaluations or performance. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. • performing process validation ensure that the process output is predictable. Medical Device Process Validation Example.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is to produce a stable. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. Process validation involves a series of activities taking place over the lifecycle of. Medical Device Process Validation Example.
From www.youtube.com
Process Validation for Medical Device Manufacturers YouTube Medical Device Process Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process verification and process validation are two important—and commonly misunderstood—activities in the development. The goal of process validation is to produce a stable. Process validation. Medical Device Process Validation Example.
From 4easyreg.com
Process Validation for Medical Devices Overview of FDA Requirements Medical Device Process Validation Example Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. As part of design and development validation, the organization shall perform clinical evaluations or performance. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is to produce. Medical Device Process Validation Example.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. The goal of process validation is to produce a stable. Process validation involves a series of activities taking place over the lifecycle of the product and process. Process verification and process validation are two important—and commonly misunderstood—activities in the development.. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device Validation Full Details PresentationEZE Medical Device Process Validation Example • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation involves a series of activities taking place over the lifecycle of the product and process. Understanding iq, oq, and pq for medical device manufacturing processes. As. Medical Device Process Validation Example.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Process Validation Example • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process verification and process validation are two important—and commonly misunderstood—activities in the development. As part of design and development validation, the. Medical Device Process Validation Example.
From www.researchgate.net
(PDF) Process Validation and Revalidation in Medical Device Production Medical Device Process Validation Example Process verification and process validation are two important—and commonly misunderstood—activities in the development. According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Understanding iq, oq, and pq for medical device manufacturing processes. The goal of process validation is to produce a stable. • performing process validation ensure that the. Medical Device Process Validation Example.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Understanding iq, oq, and. Medical Device Process Validation Example.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Device Process Validation Example • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. The goal of process validation is to produce a stable. As part of design and development validation, the organization shall perform clinical evaluations or. Medical Device Process Validation Example.
From www.youtube.com
What is Medical Device Product and Process Validation? YouTube Medical Device Process Validation Example Process validation serves as a proactive measure, identifying potential issues from failure in production before the medical. The goal of process validation is to produce a stable. Process verification and process validation are two important—and commonly misunderstood—activities in the development. Process validation involves a series of activities taking place over the lifecycle of the product and process. • performing process. Medical Device Process Validation Example.
From www.slideserve.com
PPT Medical Device Validation Process Equipment Validation Medical Device Process Validation Example As part of design and development validation, the organization shall perform clinical evaluations or performance. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Understanding iq, oq, and pq for medical device manufacturing processes. Process validation serves as a proactive measure, identifying potential issues from failure in production before the. Medical Device Process Validation Example.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Process Validation Example According to guidance published by the ghtf (now the imdrf), the following are common examples of processes that should be. Understanding iq, oq, and pq for medical device manufacturing processes. • performing process validation ensure that the process output is predictable and predetermined • the completion of appropriate process. Process validation serves as a proactive measure, identifying potential issues from. Medical Device Process Validation Example.