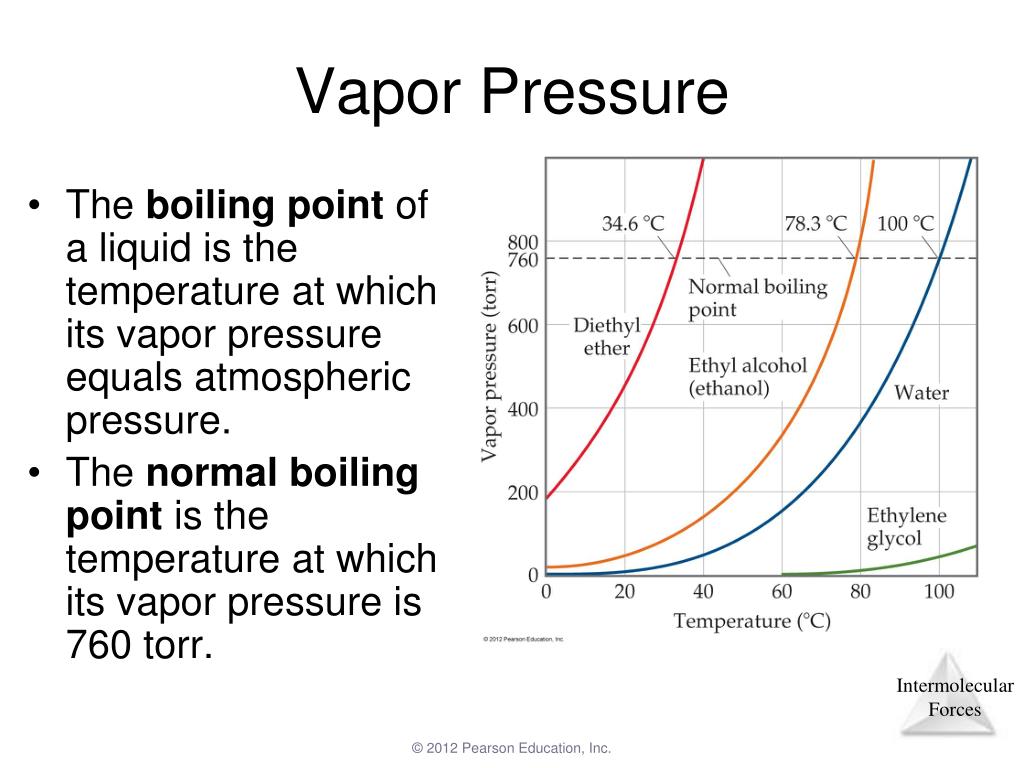
Boiling Point And Vapor Pressure Relationship . Boiling point of a substance depends upon the pressure of the system and. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. In this article we will discuss about the relationship between boiling point and vapor pressure. The web page explains the concepts of vapor. Learn how the vapor pressure and boiling point of a liquid are inversely related. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs.
from www.slideserve.com
The web page explains the concepts of vapor. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. Learn how the vapor pressure and boiling point of a liquid are inversely related. Boiling point of a substance depends upon the pressure of the system and. In this article we will discuss about the relationship between boiling point and vapor pressure.
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint
Boiling Point And Vapor Pressure Relationship The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its. In this article we will discuss about the relationship between boiling point and vapor pressure. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Learn how the vapor pressure and boiling point of a liquid are inversely related. The web page explains the concepts of vapor. Boiling point of a substance depends upon the pressure of the system and. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure.
From socratic.org
What is the relation between critical temperature and boiling point or Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. In this article we will discuss about the relationship between boiling point and vapor pressure. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. The web page. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Boiling Point And Vapor Pressure Relationship The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Boiling point of a substance depends upon the pressure of the system and. Learn how the vapor pressure and boiling point of a liquid are inversely related. Learn how intermolecular forces affect the vapor pressure and. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Vapor Pressure Normal Boiling Point & Clausius Clapeyron Equation Boiling Point And Vapor Pressure Relationship The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Boiling is the process by which a liquid turns into a vapor when. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. Boiling point of a substance depends upon the pressure of the system and. The web page explains the concepts of vapor. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the. Boiling Point And Vapor Pressure Relationship.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. In this article we will discuss about the relationship between boiling point and vapor pressure. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The point at which. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID Boiling Point And Vapor Pressure Relationship The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. In this article we will discuss about the relationship between boiling point and vapor pressure. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their. Boiling Point And Vapor Pressure Relationship.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The web page explains the concepts of vapor. The point at which the vapor pressure curve crosses the p. Boiling Point And Vapor Pressure Relationship.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Boiling Point And Vapor Pressure Relationship The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal. Boiling Point And Vapor Pressure Relationship.
From hvacrschool.com
Saturation and the PressureTemperature Relationship HVAC School Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. In this article we will discuss about the relationship between boiling point and vapor pressure. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. Boiling point of a. Boiling Point And Vapor Pressure Relationship.
From kimray.com
What's the Difference between Hydrocarbon Dew Point and Water Vapor Dew Boiling Point And Vapor Pressure Relationship Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. Boiling is the process by which a liquid turns into a vapor when it is heated to its. Boiling point of a substance depends upon the pressure of the system and. The point at which the vapor. Boiling Point And Vapor Pressure Relationship.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. The web page explains the concepts of vapor. Learn how the vapor pressure and boiling point of a liquid are inversely related. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Aim What is the relationship between vapor pressure and boiling Boiling Point And Vapor Pressure Relationship The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Boiling point of a substance depends upon the pressure of the system and. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The web page explains the concepts. Boiling Point And Vapor Pressure Relationship.
From mungfali.com
Vapor Pressure And Boiling Point Relationship Boiling Point And Vapor Pressure Relationship Learn how the vapor pressure and boiling point of a liquid are inversely related. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. In this article we will discuss about the relationship between boiling point and vapor pressure. Boiling point of a substance depends upon the. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Learn how the vapor pressure and boiling point of a liquid are inversely related. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and. Boiling Point And Vapor Pressure Relationship.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. Learn how the vapor pressure and boiling point of a liquid are inversely related. The boiling point of a liquid is the temperature at which the vapor pressure of. Boiling Point And Vapor Pressure Relationship.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Boiling Point And Vapor Pressure Relationship In this article we will discuss about the relationship between boiling point and vapor pressure. The web page explains the concepts of vapor. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids,. Boiling Point And Vapor Pressure Relationship.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Boiling point of a substance depends upon the pressure of the system and. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. In this article we will discuss about the relationship between boiling point and vapor pressure. Boiling point of a substance depends upon the pressure of the system and. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Learn. Boiling Point And Vapor Pressure Relationship.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. Boiling point of a substance depends. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Vapor Pressure and Boiling YouTube Boiling Point And Vapor Pressure Relationship The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The web page explains the concepts of vapor. Boiling is the process by. Boiling Point And Vapor Pressure Relationship.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Boiling Point And Vapor Pressure Relationship Boiling is the process by which a liquid turns into a vapor when it is heated to its. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Boiling Point And Vapor Pressure Relationship Boiling point of a substance depends upon the pressure of the system and. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The web page explains the concepts of vapor. In this article we will discuss about the relationship between boiling point and vapor pressure. Boiling. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Boiling Point And Vapor Pressure Relationship Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. Boiling point of a substance depends upon the pressure of the system and. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid.. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Boiling Point And Vapor Pressure Relationship Learn how the vapor pressure and boiling point of a liquid are inversely related. Boiling is the process by which a liquid turns into a vapor when it is heated to its. Boiling point of a substance depends upon the pressure of the system and. The point at which the vapor pressure curve crosses the p = 1 atm line. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Boiling Point And Vapor Pressure Relationship The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. In this article we will discuss about the relationship between boiling point and. Boiling Point And Vapor Pressure Relationship.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz Boiling Point And Vapor Pressure Relationship In this article we will discuss about the relationship between boiling point and vapor pressure. The web page explains the concepts of vapor. Learn how the vapor pressure and boiling point of a liquid are inversely related. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs.. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
What is the relationship between boiling point and vapor pressure Boiling Point And Vapor Pressure Relationship Boiling point of a substance depends upon the pressure of the system and. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid.. Boiling Point And Vapor Pressure Relationship.
From www.vrogue.co
Physical Equilibrium Vapor Pressure And Boiling Point vrogue.co Boiling Point And Vapor Pressure Relationship Boiling point of a substance depends upon the pressure of the system and. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the external pressure. The. Boiling Point And Vapor Pressure Relationship.
From scoop.eduncle.com
The molar enthalpy of vaporization for a liquid (normal boiling point Boiling Point And Vapor Pressure Relationship In this article we will discuss about the relationship between boiling point and vapor pressure. Learn how the vapor pressure and boiling point of a liquid are inversely related. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The boiling point of a liquid is the temperature at which the vapor. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Boiling point and external pressure YouTube Boiling Point And Vapor Pressure Relationship Boiling point of a substance depends upon the pressure of the system and. Boiling is the process by which a liquid turns into a vapor when it is heated to its. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. In this article we will. Boiling Point And Vapor Pressure Relationship.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Boiling Point And Vapor Pressure Relationship In this article we will discuss about the relationship between boiling point and vapor pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its. Learn how the vapor pressure and boiling point of a liquid are inversely related. The boiling point of a liquid is the temperature at which the vapor. Boiling Point And Vapor Pressure Relationship.
From mavink.com
Vapor Pressure And Boiling Point Relationship Boiling Point And Vapor Pressure Relationship Boiling point of a substance depends upon the pressure of the system and. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid.. Boiling Point And Vapor Pressure Relationship.
From mungfali.com
Water Boiling Point Pressure Chart Boiling Point And Vapor Pressure Relationship The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Learn how intermolecular forces affect the vapor pressure and boiling point of liquids, and how to compare different substances based on their imfs. The boiling point of a liquid is the temperature at which the vapor. Boiling Point And Vapor Pressure Relationship.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs Boiling Point And Vapor Pressure Relationship The web page explains the concepts of vapor. In this article we will discuss about the relationship between boiling point and vapor pressure. The point at which the vapor pressure curve crosses the p = 1 atm line (dashed) is the normal boiling point of the liquid. Boiling is the process by which a liquid turns into a vapor when. Boiling Point And Vapor Pressure Relationship.