What Is Orbital Number . the principal quantum number is one of three quantum numbers used to characterize an orbital. the principal quantum number tells the size and energy of the orbital. All the orbitals of ‘n’ contains a single shell of an. Within each shell of an. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. An atomic orbital , which is distinct. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. the angular quantum number (l) describes the shape of the orbital.
from www.sciencefacts.net
the principal quantum number tells the size and energy of the orbital. the principal quantum number is one of three quantum numbers used to characterize an orbital. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. the angular quantum number (l) describes the shape of the orbital. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Within each shell of an. An atomic orbital , which is distinct. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. All the orbitals of ‘n’ contains a single shell of an. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).
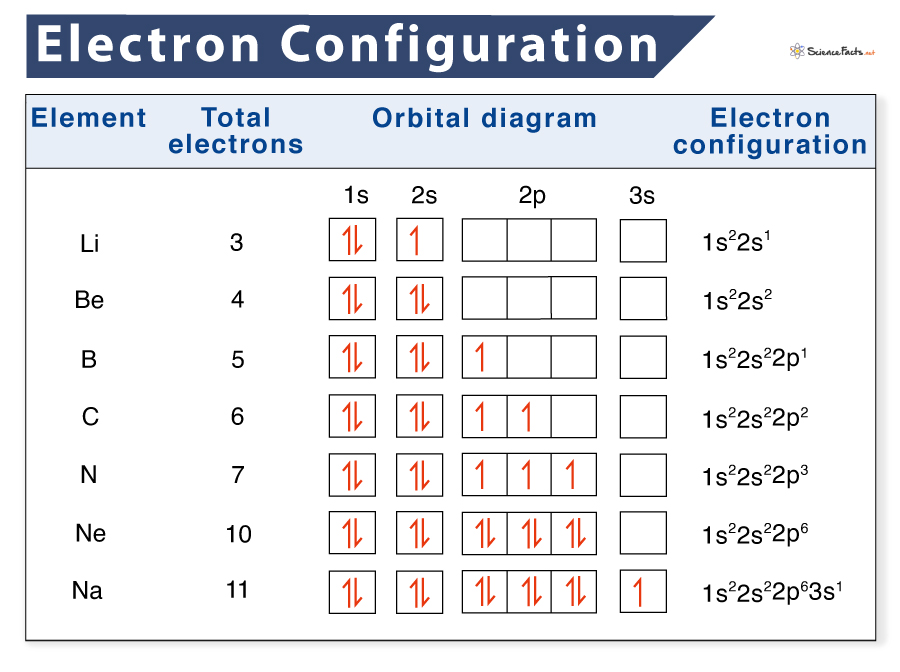
Electron Configuration Definition, Examples, Chart, and Diagram
What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the angular quantum number (l) describes the shape of the orbital. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. All the orbitals of ‘n’ contains a single shell of an. the principal quantum number tells the size and energy of the orbital. the principal quantum number is one of three quantum numbers used to characterize an orbital. Within each shell of an. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). An atomic orbital , which is distinct.
From www.vrogue.co
Quantum Number Orbital Definition Formula Diagram Sha vrogue.co What Is Orbital Number Within each shell of an. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the principal quantum number tells the size and energy of the orbital. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. 12 rows there. What Is Orbital Number.
From chem.libretexts.org
2.2 Atomic Orbitals and Quantum Numbers Chemistry LibreTexts What Is Orbital Number Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. An atomic orbital , which is distinct. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. All the orbitals of ‘n’ contains a single shell of an. in quantum. What Is Orbital Number.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is Orbital Number each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. Within each shell of an. All the orbitals of ‘n’ contains a single shell of an. the angular quantum number (l) describes the shape of the orbital. An atomic orbital , which is distinct. the principal quantum number. What Is Orbital Number.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Presentation ID7079563 What Is Orbital Number in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the principal quantum number tells the size and energy of the orbital. Orbitals have shapes that. What Is Orbital Number.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). All the orbitals of ‘n’ contains a single shell of an. the principal quantum number is one of three quantum numbers used to characterize an orbital. Within each shell of an. the angular. What Is Orbital Number.
From www.vedantu.com
Shapes of Orbitals What is Orbital? Types of Orbitals What Is Orbital Number All the orbitals of ‘n’ contains a single shell of an. the angular quantum number (l) describes the shape of the orbital. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the principal quantum number is one of three quantum numbers used to characterize an orbital. Orbitals have shapes. What Is Orbital Number.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Is Orbital Number the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. the principal quantum number is one of three quantum numbers used to characterize an orbital. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).. What Is Orbital Number.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID6909895 What Is Orbital Number the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. the angular quantum number (l) describes the shape of the orbital. All the orbitals of ‘n’ contains a single shell of. What Is Orbital Number.
From stock.adobe.com
Electron configuration notation. Type of orbital. Number of electrons in orbital. Energy level What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. Within each shell of an. the angular quantum number (l) describes the shape of the. What Is Orbital Number.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is Orbital Number An atomic orbital , which is distinct. the principal quantum number is one of three quantum numbers used to characterize an orbital. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.. What Is Orbital Number.
From www.showme.com
Orbital Notation (watch after Electron Configuration) Science, Chemistry ShowMe What Is Orbital Number each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. the principal quantum number tells the size and energy of the orbital. the angular quantum number (l) describes the shape of the orbital. the principal quantum number is one of three quantum numbers used to characterize an. What Is Orbital Number.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. the principal quantum number is one of three quantum numbers used to characterize an orbital.. What Is Orbital Number.
From schematiclistpact101.z22.web.core.windows.net
How To Read Orbital Diagrams What Is Orbital Number Within each shell of an. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. the angular quantum number (l) describes the shape of the orbital. An atomic orbital ,. What Is Orbital Number.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook What Is Orbital Number Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. An atomic orbital , which is distinct. the number 1 represents the fact that the orbital is in the energy. What Is Orbital Number.
From www.alamy.com
Electron configuration notation. Type of orbital. Number of electrons in orbital. Energy level What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of. What Is Orbital Number.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Practice Problems YouTube What Is Orbital Number An atomic orbital , which is distinct. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the principal quantum number is one of three quantum. What Is Orbital Number.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. the angular quantum number (l) describes the shape of the orbital. each wavefunction with. What Is Orbital Number.
From en.wikipedia.org
FileOrbitals table.png Wikipedia What Is Orbital Number Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. An atomic orbital , which is distinct. each wavefunction with an allowed combination of n, l, and m l values describes. What Is Orbital Number.
From www.albert.io
Quantum Numbers and Theory AP® Chemistry Crash Course Albert.io What Is Orbital Number An atomic orbital , which is distinct. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. All the orbitals of ‘n’ contains a single shell of an. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. 12 rows there. What Is Orbital Number.
From icdsc.org
How many electrons are in each shell including 3p orbitals What Is Orbital Number the angular quantum number (l) describes the shape of the orbital. each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. the principal quantum number tells the size and energy of the orbital. 12 rows there are four types of orbitals that you should be familiar with. What Is Orbital Number.
From chemwiki.ucdavis.edu
Electronic Orbitals Chemwiki What Is Orbital Number the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. Within each shell of an. An atomic orbital , which is distinct. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. All the orbitals of ‘n’ contains a single shell of an.. What Is Orbital Number.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of. What Is Orbital Number.
From www.britannica.com
Electronic configuration Definition, Orbitals, & Facts Britannica What Is Orbital Number Within each shell of an. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). All the orbitals of ‘n’ contains a single shell of an. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. . What Is Orbital Number.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). All the orbitals of ‘n’ contains a single shell of an. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. each wavefunction with an. What Is Orbital Number.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states. What Is Orbital Number.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is Orbital Number the principal quantum number is one of three quantum numbers used to characterize an orbital. the angular quantum number (l) describes the shape of the orbital. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. An atomic orbital , which is distinct. the number 1 represents the fact. What Is Orbital Number.
From www.slideserve.com
PPT Electron Configuration and the Periodic Table PowerPoint Presentation ID2050406 What Is Orbital Number the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle,. What Is Orbital Number.
From www.thoughtco.com
Electron Configuration Chart What Is Orbital Number All the orbitals of ‘n’ contains a single shell of an. the principal quantum number tells the size and energy of the orbital. the principal quantum number is one of three quantum numbers used to characterize an orbital. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f. What Is Orbital Number.
From mavink.com
Quantum Numbers Orbitals Chart What Is Orbital Number the principal quantum number is one of three quantum numbers used to characterize an orbital. Within each shell of an. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. . What Is Orbital Number.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is Orbital Number each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the number 1 represents the fact that the orbital is in the energy level. What Is Orbital Number.
From socratic.org
Which are the orbitals(s,p,d,f) have center of symmetry? Socratic What Is Orbital Number each wavefunction with an allowed combination of n, l, and m l values describes an atomic orbital, a particular. the principal quantum number tells the size and energy of the orbital. in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the number 1 represents the fact that the. What Is Orbital Number.
From lucillemeowdurham.blogspot.com
The Size of an Atomic Orbital Is Associated With What Is Orbital Number in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. the principal quantum number is one of three quantum numbers used to characterize an orbital. An atomic orbital , which is distinct.. What Is Orbital Number.
From www.pathwaystochemistry.com
Quantum Mechanics Quantum Numbers and Orbitals Pathways to Chemistry What Is Orbital Number An atomic orbital , which is distinct. Orbitals have shapes that are best described as spherical ( l = 0), polar ( l = 1), or. the number 1 represents the fact that the orbital is in the energy level closest to the nucleus. the angular quantum number (l) describes the shape of the orbital. the principal. What Is Orbital Number.
From polizhuge.weebly.com
Atomic orbitals explained polizhuge What Is Orbital Number All the orbitals of ‘n’ contains a single shell of an. the principal quantum number tells the size and energy of the orbital. An atomic orbital , which is distinct. the principal quantum number is one of three quantum numbers used to characterize an orbital. each wavefunction with an allowed combination of n, l, and m l. What Is Orbital Number.
From saylordotorg.github.io
Quantum Numbers for Electrons What Is Orbital Number 12 rows there are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). the angular quantum number (l) describes the shape of the orbital. Within each shell of an. All the orbitals of ‘n’ contains a single shell of an. the principal quantum number is one. What Is Orbital Number.