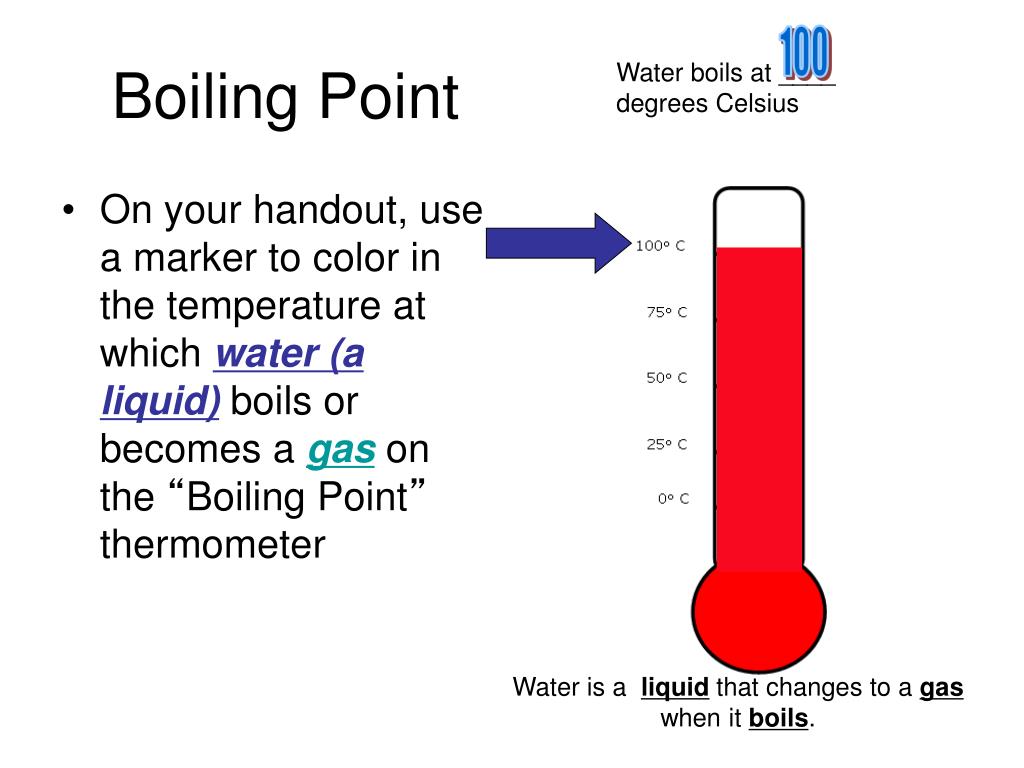
How Does Salt Affect Water Boiling Point . The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. The tale is true, but the difference is negligible, an expert. However, taking the solute out of the water and putting it in the gas phase (air) requires. Salt increases the boiling point and decreases the melting point of water. If the water level has gone below 400 ml , pour a little. The boiling point of water increases when salt is dissolved in it. Observe the change in temperature as it rises to a new boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by. Why does salt affect boiling point? Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. This phenomenon is known as boiling point elevation. Salt (or other solutes, like sugar) can easily dissolve in liquid water. That means if you add salt before heating some water on the stove, it will.
from spikaph.blogspot.com
Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. Salt (or other solutes, like sugar) can easily dissolve in liquid water. Why does salt affect boiling point? That means if you add salt before heating some water on the stove, it will. The boiling point of water increases when salt is dissolved in it. The temperature needed to boil will increase by. Observe the change in temperature as it rises to a new boiling point. Salt is sodium chloride, which is an ionic. Salt increases the boiling point and decreases the melting point of water. If you add salt to water, you raise the boiling point, or the temperature at which water boils.
Boiling Point Of Water Boiling point of water YouTube For
How Does Salt Affect Water Boiling Point Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. That means if you add salt before heating some water on the stove, it will. Salt (or other solutes, like sugar) can easily dissolve in liquid water. Observe the change in temperature as it rises to a new boiling point. If the water level has gone below 400 ml , pour a little. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. This phenomenon is known as boiling point elevation. If you add salt to water, you raise the boiling point, or the temperature at which water boils. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. However, taking the solute out of the water and putting it in the gas phase (air) requires. Why does salt affect boiling point? Salt increases the boiling point and decreases the melting point of water. The temperature needed to boil will increase by. The boiling point of water increases when salt is dissolved in it. The tale is true, but the difference is negligible, an expert.
From thevigyan.com
Why does adding salt increase water’s boiling point? The Vigyan How Does Salt Affect Water Boiling Point The boiling point of water increases when salt is dissolved in it. This phenomenon is known as boiling point elevation. The tale is true, but the difference is negligible, an expert. That means if you add salt before heating some water on the stove, it will. Why does salt affect boiling point? The fact that dissolving a salt in a. How Does Salt Affect Water Boiling Point.
From reportd731.web.fc2.com
Science Lab to Determine the Rise in Boiling Point of Water When Adding How Does Salt Affect Water Boiling Point Why does salt affect boiling point? Salt (or other solutes, like sugar) can easily dissolve in liquid water. If the water level has gone below 400 ml , pour a little. Salt is sodium chloride, which is an ionic. However, taking the solute out of the water and putting it in the gas phase (air) requires. This phenomenon is known. How Does Salt Affect Water Boiling Point.
From www.youtube.com
What AFFECT does salt have on the boiling point of water YouTube How Does Salt Affect Water Boiling Point Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. This phenomenon is known as boiling point elevation. However, taking the solute out of the water and putting it in the gas phase (air) requires. The temperature needed to boil will increase by. Why does salt affect boiling point? Salt is sodium. How Does Salt Affect Water Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? How Does Salt Affect Water Boiling Point If the water level has gone below 400 ml , pour a little. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Salt is sodium chloride, which is an ionic. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. The tale is. How Does Salt Affect Water Boiling Point.
From childhealthpolicy.vumc.org
😝 Does salt affect the boiling point of water experiment. What is the How Does Salt Affect Water Boiling Point Salt increases the boiling point and decreases the melting point of water. If the water level has gone below 400 ml , pour a little. The temperature needed to boil will increase by. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. Salt (or other. How Does Salt Affect Water Boiling Point.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For How Does Salt Affect Water Boiling Point The boiling point of water increases when salt is dissolved in it. If the water level has gone below 400 ml , pour a little. The temperature needed to boil will increase by. Salt (or other solutes, like sugar) can easily dissolve in liquid water. That means if you add salt before heating some water on the stove, it will.. How Does Salt Affect Water Boiling Point.
From learningschoolcargeese.z14.web.core.windows.net
Adding Salt In Water Boiling Point How Does Salt Affect Water Boiling Point The tale is true, but the difference is negligible, an expert. This phenomenon is known as boiling point elevation. If the water level has gone below 400 ml , pour a little. That means if you add salt before heating some water on the stove, it will. Why does salt affect boiling point? If you add salt to water, you. How Does Salt Affect Water Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy How Does Salt Affect Water Boiling Point Salt is sodium chloride, which is an ionic. Salt increases the boiling point and decreases the melting point of water. Observe the change in temperature as it rises to a new boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. That means if you add salt before heating some. How Does Salt Affect Water Boiling Point.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube How Does Salt Affect Water Boiling Point However, taking the solute out of the water and putting it in the gas phase (air) requires. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. Observe the change in temperature as it rises to a new boiling point. Salt is sodium chloride, which is. How Does Salt Affect Water Boiling Point.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point How Does Salt Affect Water Boiling Point Observe the change in temperature as it rises to a new boiling point. If the water level has gone below 400 ml , pour a little. That means if you add salt before heating some water on the stove, it will. The boiling point of water increases when salt is dissolved in it. The fact that dissolving a salt in. How Does Salt Affect Water Boiling Point.
From www.thoughtco.com
Why Does Adding Salt Increase the Boiling Point of Water? How Does Salt Affect Water Boiling Point The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If the water level has gone below 400 ml , pour a little. According to an old wives'. How Does Salt Affect Water Boiling Point.
From facts.net
20 Fascinating Facts About Boiling Point Elevation How Does Salt Affect Water Boiling Point The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. The tale is true, but the difference is negligible, an expert. However, taking the solute out of the water and putting it in the gas phase (air) requires. Observe the change in temperature as it rises. How Does Salt Affect Water Boiling Point.
From klahkiyli.blob.core.windows.net
What Is Boiling Point Of Salt Water at Tracy Mendoza blog How Does Salt Affect Water Boiling Point According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. That means if you add salt before heating some water on the stove, it will. This phenomenon is known as boiling point elevation. The tale is true, but the difference is negligible, an expert. The fact that dissolving a. How Does Salt Affect Water Boiling Point.
From ar.inspiredpencil.com
Boiling Point Of Water Graph How Does Salt Affect Water Boiling Point Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. The temperature needed to boil will increase by. Salt (or other solutes, like sugar) can easily dissolve in liquid water. This phenomenon is known as boiling point elevation. The tale is true, but the difference is negligible, an expert. According to an. How Does Salt Affect Water Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples How Does Salt Affect Water Boiling Point If you add salt to water, you raise the boiling point, or the temperature at which water boils. Salt is sodium chloride, which is an ionic. That means if you add salt before heating some water on the stove, it will. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the. How Does Salt Affect Water Boiling Point.
From childhealthpolicy.vumc.org
😝 Does salt affect the boiling point of water experiment. What is the How Does Salt Affect Water Boiling Point Salt (or other solutes, like sugar) can easily dissolve in liquid water. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. The tale is true, but the difference is negligible, an expert. If you add salt to water, you raise the boiling point, or the temperature at which. How Does Salt Affect Water Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart How Does Salt Affect Water Boiling Point If the water level has gone below 400 ml , pour a little. That means if you add salt before heating some water on the stove, it will. Salt increases the boiling point and decreases the melting point of water. This phenomenon is known as boiling point elevation. According to an old wives' tale, adding salt to a pot of. How Does Salt Affect Water Boiling Point.
From www.youtube.com
How does salt affect the freezing and boiling points of water? YouTube How Does Salt Affect Water Boiling Point Observe the change in temperature as it rises to a new boiling point. Salt is sodium chloride, which is an ionic. If the water level has gone below 400 ml , pour a little. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. If you add salt to water, you raise. How Does Salt Affect Water Boiling Point.
From klahkiyli.blob.core.windows.net
What Is Boiling Point Of Salt Water at Tracy Mendoza blog How Does Salt Affect Water Boiling Point Salt increases the boiling point and decreases the melting point of water. The temperature needed to boil will increase by. Observe the change in temperature as it rises to a new boiling point. Why does salt affect boiling point? Salt (or other solutes, like sugar) can easily dissolve in liquid water. Salt is sodium chloride, which is an ionic. According. How Does Salt Affect Water Boiling Point.
From nerdyseal.com
How Does Salt Affect the Boiling Point of Water? Essay Sample 575 How Does Salt Affect Water Boiling Point The boiling point of water increases when salt is dissolved in it. That means if you add salt before heating some water on the stove, it will. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. Salt is sodium chloride, which is an ionic. However, taking the solute out of the. How Does Salt Affect Water Boiling Point.
From snipe.fm
😍 How does salt affect boiling point. Why Does Adding Salt Increase the How Does Salt Affect Water Boiling Point If you add salt to water, you raise the boiling point, or the temperature at which water boils. Salt is sodium chloride, which is an ionic. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. Salt increases the boiling point and decreases the melting point of water. The. How Does Salt Affect Water Boiling Point.
From www.perkinselearning.org
Can the Boiling Point of Water be Changed? Perkins eLearning How Does Salt Affect Water Boiling Point The tale is true, but the difference is negligible, an expert. If the water level has gone below 400 ml , pour a little. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. Salt increases the boiling point and decreases the melting point of water. However, taking the. How Does Salt Affect Water Boiling Point.
From www.thoughtco.com
Does Adding Salt Lower the Boiling Point of Water? How Does Salt Affect Water Boiling Point Why does salt affect boiling point? However, taking the solute out of the water and putting it in the gas phase (air) requires. This phenomenon is known as boiling point elevation. Observe the change in temperature as it rises to a new boiling point. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point. How Does Salt Affect Water Boiling Point.
From mlsciencefair.com
How Salt Affects Boiling Point of Water Maple Leaf Science Fair How Does Salt Affect Water Boiling Point According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. If you add salt to water, you raise the boiling point, or the temperature at. How Does Salt Affect Water Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts How Does Salt Affect Water Boiling Point However, taking the solute out of the water and putting it in the gas phase (air) requires. The temperature needed to boil will increase by. The tale is true, but the difference is negligible, an expert. Salt increases the boiling point and decreases the melting point of water. Salt (or other solutes, like sugar) can easily dissolve in liquid water.. How Does Salt Affect Water Boiling Point.
From www.animalia-life.club
Boiling Point Of Water Examples How Does Salt Affect Water Boiling Point The tale is true, but the difference is negligible, an expert. This phenomenon is known as boiling point elevation. Why does salt affect boiling point? Salt is sodium chloride, which is an ionic. Salt increases the boiling point and decreases the melting point of water. Observe the change in temperature as it rises to a new boiling point. However, taking. How Does Salt Affect Water Boiling Point.
From howchimp.com
Does Salt Make Water Boil Faster? [Adding Salt Time to Boil] HowChimp How Does Salt Affect Water Boiling Point This phenomenon is known as boiling point elevation. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. The temperature needed to boil will increase by. That means. How Does Salt Affect Water Boiling Point.
From chart-studio.plotly.com
How Does Salt Affect the Boiling Point of Water? bar chart made by How Does Salt Affect Water Boiling Point Salt is sodium chloride, which is an ionic. The boiling point of water increases when salt is dissolved in it. Observe the change in temperature as it rises to a new boiling point. The temperature needed to boil will increase by. This phenomenon is known as boiling point elevation. Why does salt affect boiling point? That means if you add. How Does Salt Affect Water Boiling Point.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For How Does Salt Affect Water Boiling Point Why does salt affect boiling point? However, taking the solute out of the water and putting it in the gas phase (air) requires. The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. Basically, the amount of salt people add to water for cooking doesn't affect. How Does Salt Affect Water Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills How Does Salt Affect Water Boiling Point Why does salt affect boiling point? However, taking the solute out of the water and putting it in the gas phase (air) requires. That means if you add salt before heating some water on the stove, it will. Observe the change in temperature as it rises to a new boiling point. The tale is true, but the difference is negligible,. How Does Salt Affect Water Boiling Point.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy How Does Salt Affect Water Boiling Point The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. If the water level has gone below 400 ml , pour a little. According to an old wives' tale, adding salt to a pot of water on the stove will make it boil faster. This phenomenon. How Does Salt Affect Water Boiling Point.
From thermtest.com
Does Adding Salt to Water Help it Boil Faster? and Why? How Does Salt Affect Water Boiling Point That means if you add salt before heating some water on the stove, it will. Salt (or other solutes, like sugar) can easily dissolve in liquid water. Observe the change in temperature as it rises to a new boiling point. The temperature needed to boil will increase by. If you add salt to water, you raise the boiling point, or. How Does Salt Affect Water Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Salt Affect Water Boiling Point The fact that dissolving a salt in a liquid, such as water, affects its boiling point comes under the general heading of colligative properties. Salt (or other solutes, like sugar) can easily dissolve in liquid water. The boiling point of water increases when salt is dissolved in it. The temperature needed to boil will increase by. However, taking the solute. How Does Salt Affect Water Boiling Point.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For How Does Salt Affect Water Boiling Point That means if you add salt before heating some water on the stove, it will. Basically, the amount of salt people add to water for cooking doesn't affect the boiling point at all. Salt is sodium chloride, which is an ionic. Why does salt affect boiling point? However, taking the solute out of the water and putting it in the. How Does Salt Affect Water Boiling Point.
From www.cookist.com
Why Do Some People Add Salt To Boiling Water? How Does Salt Affect Water Boiling Point The boiling point of water increases when salt is dissolved in it. The tale is true, but the difference is negligible, an expert. Salt (or other solutes, like sugar) can easily dissolve in liquid water. That means if you add salt before heating some water on the stove, it will. Salt is sodium chloride, which is an ionic. According to. How Does Salt Affect Water Boiling Point.