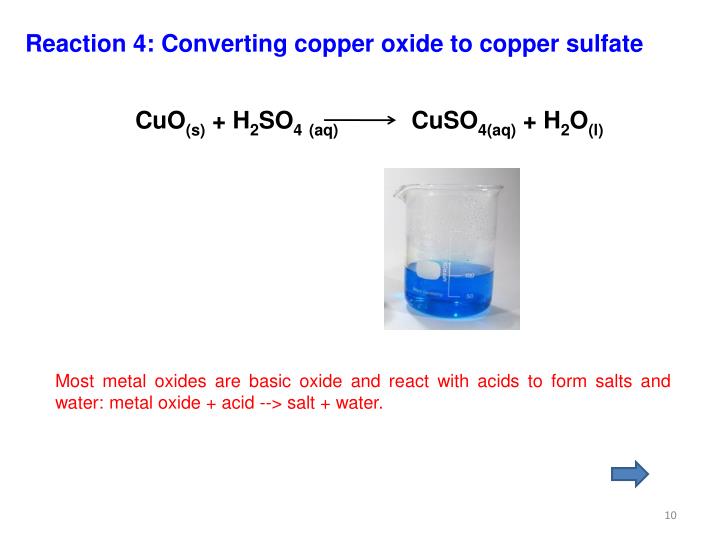
Copper Sulfate Vs Copper Sulfide . The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. The toxicity of copper sulfate depends on the copper. Those containing only two elements, the principal. As with other elements, the simplest compounds of copper are binary compounds, i.e. Copper sulfate is an inorganic compound that combines sulfur with copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. It can kill bacteria, algae, roots, plants, snails, and fungi. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and.
from www.slideserve.com
Those containing only two elements, the principal. As with other elements, the simplest compounds of copper are binary compounds, i.e. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. It can kill bacteria, algae, roots, plants, snails, and fungi. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The toxicity of copper sulfate depends on the copper. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. Copper sulfate is an inorganic compound that combines sulfur with copper.
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint
Copper Sulfate Vs Copper Sulfide The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. It can kill bacteria, algae, roots, plants, snails, and fungi. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. Copper sulfate is an inorganic compound that combines sulfur with copper. The toxicity of copper sulfate depends on the copper. As with other elements, the simplest compounds of copper are binary compounds, i.e. Those containing only two elements, the principal.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper Sulfate Vs Copper Sulfide The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. The toxicity of copper sulfate depends on the copper. It can kill bacteria, algae, roots, plants, snails, and fungi. The differences in crystal structure between copper sulfides lead to differences in. Copper Sulfate Vs Copper Sulfide.
From chemistrylabs-2.blogspot.com
Electrolysis Of Copper Sulfate Solution Chemistry Labs Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. The toxicity of copper sulfate depends on the copper. Copper sulfate is an inorganic compound that combines sulfur with copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due. Copper Sulfate Vs Copper Sulfide.
From www.researchgate.net
Temperature dependence of the solubility of copper sulfate in water. Download Scientific Diagram Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The differences in crystal structure between copper sulfides. Copper Sulfate Vs Copper Sulfide.
From www.sciencephoto.com
Copper Sulphate Stock Image C028/1275 Science Photo Library Copper Sulfate Vs Copper Sulfide As with other elements, the simplest compounds of copper are binary compounds, i.e. It can kill bacteria, algae, roots, plants, snails, and fungi. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
COPPER SULPHATE USES BENEFITS (कॉपर सल्फेट) YouTube Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. As with. Copper Sulfate Vs Copper Sulfide.
From www.sciencephoto.com
Formation of copper sulphate crystals, 3 of 3 Stock Image C036/3511 Science Photo Library Copper Sulfate Vs Copper Sulfide As with other elements, the simplest compounds of copper are binary compounds, i.e. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. Those containing only two elements, the principal. This article discusses the benefits of copper sulfate and the side effects that can come from copper. Copper Sulfate Vs Copper Sulfide.
From www.suchemind.com
CopperSulphateCopperSulfate Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a. Copper Sulfate Vs Copper Sulfide.
From fphoto.photoshelter.com
science chemistry compound cupric sulfate Fundamental Photographs The Art of Science Copper Sulfate Vs Copper Sulfide It can kill bacteria, algae, roots, plants, snails, and fungi. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. Those containing only two elements, the principal. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a. Copper Sulfate Vs Copper Sulfide.
From testbook.com
Copper Sulphate Formula Structure, Preparation, Properties & Uses Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. As with other elements, the simplest compounds of copper are binary compounds, i.e. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. The toxicity of copper sulfate depends on the copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
Double displacement Na2S + CuSO4 Sodium sulphide + Copper sulphate Precipitation reaction Copper Sulfate Vs Copper Sulfide The toxicity of copper sulfate depends on the copper. As with other elements, the simplest compounds of copper are binary compounds, i.e. Copper sulfate is an inorganic compound that combines sulfur with copper. It can kill bacteria, algae, roots, plants, snails, and fungi. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get. Copper Sulfate Vs Copper Sulfide.
From www.slideserve.com
PPT CHAPTER 6 PHYSICAL AND CHEMICAL CHANGES PowerPoint Presentation ID630309 Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. It can kill bacteria, algae, roots, plants, snails, and fungi. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. Copper sulfate is an inorganic compound that combines sulfur. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
Hydrating Anhydrous Copper Sulphate, Chemical Reaction YouTube Copper Sulfate Vs Copper Sulfide The toxicity of copper sulfate depends on the copper. Those containing only two elements, the principal. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. As with other elements, the simplest compounds of copper. Copper Sulfate Vs Copper Sulfide.
From www.sciencephoto.com
Hydrated and Anhydrous Copper Sulfate Stock Image C022/1869 Science Photo Library Copper Sulfate Vs Copper Sulfide The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. Copper sulfate is an inorganic compound that combines sulfur with copper. The toxicity of copper sulfate depends on the copper. For example, if you react copper(i) oxide with hot dilute sulfuric. Copper Sulfate Vs Copper Sulfide.
From pixels.com
Anhydrous Copper (ii) Sulphate Photograph by Martyn F. Chillmaid/science Photo Library Pixels Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. Those containing only two elements, the principal. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. As with. Copper Sulfate Vs Copper Sulfide.
From amizaraspecialitychemicals.co.in
Understanding the Uses and Benefits of Copper Sulphate in Agriculture Amizara Copper Sulfate Vs Copper Sulfide This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. As with other elements, the simplest compounds of copper are binary compounds, i.e. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. It can kill bacteria, algae, roots,. Copper Sulfate Vs Copper Sulfide.
From ar.inspiredpencil.com
Copper Sulfate Solution Copper Sulfate Vs Copper Sulfide It can kill bacteria, algae, roots, plants, snails, and fungi. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. Those containing only two. Copper Sulfate Vs Copper Sulfide.
From ar.inspiredpencil.com
Copper Sulfate Aqueous Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. Copper sulfate is an inorganic compound that combines sulfur with copper. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The toxicity of copper sulfate depends on the copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due. Copper Sulfate Vs Copper Sulfide.
From www.goldhilleducation.co.uk
IGCSE Chemistry Mychem Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. Copper sulfate is an inorganic compound that combines sulfur with copper. The toxicity. Copper Sulfate Vs Copper Sulfide.
From imbibe-solutions.com
The NotSoStinky Perks of Sulfur — Imbibe Solutions Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. Those containing only two elements, the principal. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. This article discusses the benefits of copper sulfate and the side effects that. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
Group 2 Salt Analysis Copper Sulphate salt Chemistry Practical Class 11 and 12 YouTube Copper Sulfate Vs Copper Sulfide This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. The toxicity of copper sulfate depends on the copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. Those containing only two. Copper Sulfate Vs Copper Sulfide.
From www.sciencephoto.com
Hydrated copper sulphate crystals Stock Image C001/9285 Science Photo Library Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. It can kill bacteria, algae, roots, plants, snails, and fungi. Copper sulfate is an inorganic compound that combines sulfur with copper. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation. Copper Sulfate Vs Copper Sulfide.
From ar.inspiredpencil.com
Anhydrous Copper Sulphate Copper Sulfate Vs Copper Sulfide This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. Those containing. Copper Sulfate Vs Copper Sulfide.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Sulphate Copper Sulfate Vs Copper Sulfide The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The toxicity of copper sulfate depends on the copper. Those containing only two elements, the principal. The difference is that. Copper Sulfate Vs Copper Sulfide.
From hbsyplastic.en.made-in-china.com
Copper Sulphate Pentahydrate Feed 99 Anhydrous Copper Sulfate Copper Sulfate and Cupric Sulfate Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. The toxicity of copper sulfate depends on the copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. This article discusses the benefits of copper sulfate and the side. Copper Sulfate Vs Copper Sulfide.
From www.katyayaniorganics.com
Katyayani Copper Sulphate Fungicide Katyayani Organics Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. It can kill bacteria, algae, roots, plants, snails, and fungi. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i). Copper Sulfate Vs Copper Sulfide.
From sciencekitstore.com
Copper Sulfate, Pentahydrate High Purity Chemical for Diverse Applications Copper Sulfate Vs Copper Sulfide As with other elements, the simplest compounds of copper are binary compounds, i.e. Those containing only two elements, the principal. It can kill bacteria, algae, roots, plants, snails, and fungi. Copper sulfate is an inorganic compound that combines sulfur with copper. The toxicity of copper sulfate depends on the copper. This article discusses the benefits of copper sulfate and the. Copper Sulfate Vs Copper Sulfide.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Sulfate Vs Copper Sulfide It can kill bacteria, algae, roots, plants, snails, and fungi. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. Copper sulfate is an inorganic compound that combines sulfur. Copper Sulfate Vs Copper Sulfide.
From melscience.com
Copper(II) sulfate MEL Chemistry Copper Sulfate Vs Copper Sulfide It can kill bacteria, algae, roots, plants, snails, and fungi. Those containing only two elements, the principal. This article discusses the benefits of copper sulfate and the side effects that can come from copper sulfate. As with other elements, the simplest compounds of copper are binary compounds, i.e. Copper sulfate is an inorganic compound that combines sulfur with copper. The. Copper Sulfate Vs Copper Sulfide.
From www.sciencephoto.com
Copper (II) Sulphate Stock Image C036/3376 Science Photo Library Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. As with other elements, the simplest compounds of copper are binary compounds, i.e. It can kill bacteria, algae, roots, plants,. Copper Sulfate Vs Copper Sulfide.
From www.animalia-life.club
Crystallisation Of Copper Sulphate Copper Sulfate Vs Copper Sulfide The toxicity of copper sulfate depends on the copper. Copper sulfate is an inorganic compound that combines sulfur with copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in its structure (for same reason ferrous sulphate has a green colour), while. It can kill bacteria, algae, roots, plants, snails, and fungi. As. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
How to Write the Formula for Copper (II) sulfide YouTube Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. As with other elements, the simplest compounds of copper are binary compounds, i.e. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due to the presence of water molecules in. Copper Sulfate Vs Copper Sulfide.
From insanitek.net
The Many Uses for Copper Sulphate Around the Home and in the Science Lab Insanitek Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. Those containing only two elements, the principal. As with other elements, the simplest compounds of copper are binary compounds, i.e.. Copper Sulfate Vs Copper Sulfide.
From maguirehq.com
Copper Sulphate Maguires Copper Sulfate Vs Copper Sulfide Copper sulfate is an inorganic compound that combines sulfur with copper. The differences in crystal structure between copper sulfides lead to differences in dissolution, oxidative, and flotation behaviors. As with other elements, the simplest compounds of copper are binary compounds, i.e. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution. Copper Sulfate Vs Copper Sulfide.
From www.youtube.com
Hydrated and anhydrous copper sulphate YouTube Copper Sulfate Vs Copper Sulfide Those containing only two elements, the principal. For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. The toxicity of copper sulfate depends on the copper. Copper sulfate is an inorganic compound that combines sulfur with copper. The difference is that $\ce{cuso4.5h2o}$ has a blue colour due. Copper Sulfate Vs Copper Sulfide.
From www.laboratorynotes.com
Copper(II) Sulfate Pentahydrate Pentahydrate (Blue Vitriol) [CuSO4.5H2O] Molecular Weight Copper Sulfate Vs Copper Sulfide For example, if you react copper(i) oxide with hot dilute sulfuric acid, you might expect to get a solution of copper(i) sulfate and. As with other elements, the simplest compounds of copper are binary compounds, i.e. Those containing only two elements, the principal. It can kill bacteria, algae, roots, plants, snails, and fungi. Copper sulfate is an inorganic compound that. Copper Sulfate Vs Copper Sulfide.