Can K Form A Negative Ion . if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. nonmetals form negative ions (anions). remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must gain three electrons to have the same number of electrons as an. remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. A proton never moves from one atom to another. Naming ions and ionic compounds. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. High school chemistry > unit 3.
from general.chemistrysteps.com
Naming ions and ionic compounds. A proton never moves from one atom to another. remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to. nonmetals form negative ions (anions). a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. High school chemistry > unit 3. remember that ions are formed only when electrons move from one atom to another;
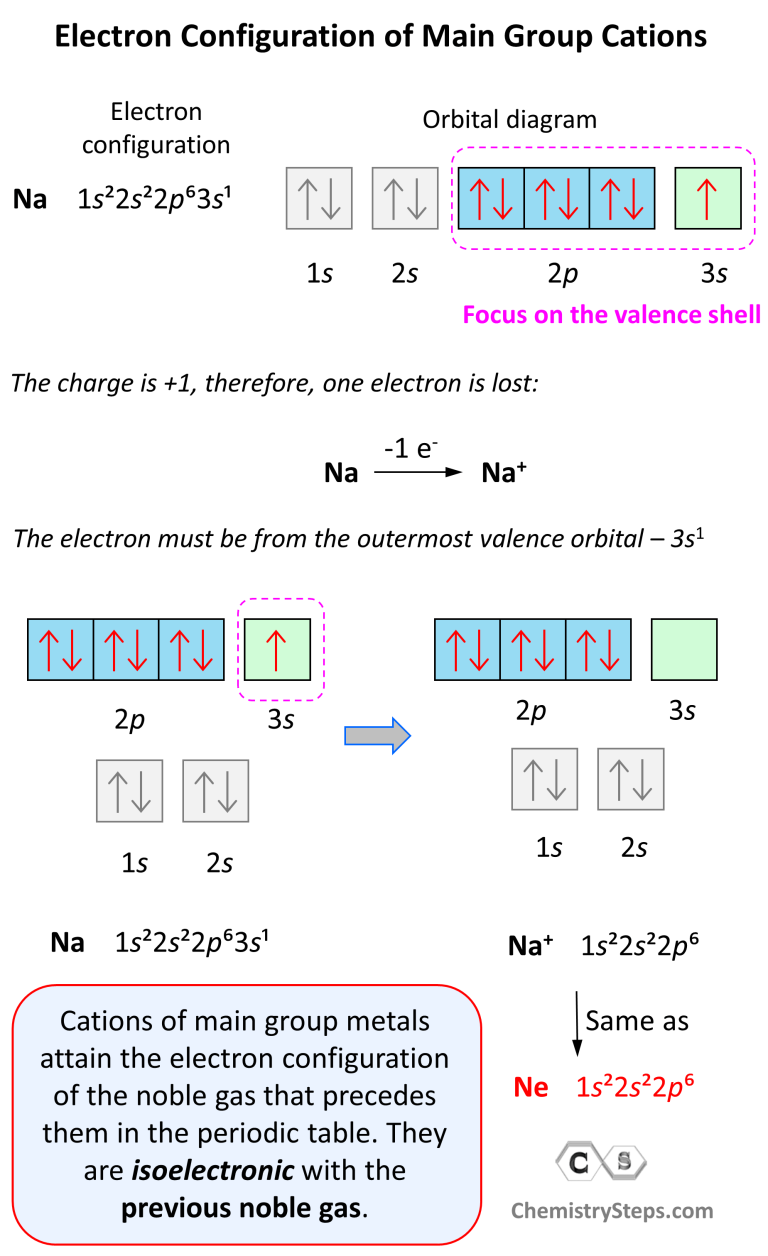
Electron Configurations of Ions Chemistry Steps
Can K Form A Negative Ion remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. High school chemistry > unit 3. A proton never moves from one atom to another. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. remember that ions are formed only when electrons move from one atom to another; Naming ions and ionic compounds. remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. A proton never moves from one atom to. A nitrogen atom must gain three electrons to have the same number of electrons as an. nonmetals form negative ions (anions).
From learningschoolns1i1zoiu.z22.web.core.windows.net
How To Name Ions In Chemistry Can K Form A Negative Ion A proton never moves from one atom to another. remember that ions are formed only when electrons move from one atom to another; if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which. Can K Form A Negative Ion.
From exodvnood.blob.core.windows.net
Can Neon Form A Negative Ion at Zaida Wright blog Can K Form A Negative Ion nonmetals form negative ions (anions). remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must gain three electrons to have the same number of electrons as an. A proton never moves from one atom to. remember that ions are formed only when electrons move from one atom to another;. Can K Form A Negative Ion.
From www.slideserve.com
PPT Ions PowerPoint Presentation, free download ID6738771 Can K Form A Negative Ion A proton never moves from one atom to another. nonmetals form negative ions (anions). a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. A proton never moves from one atom to. High school chemistry > unit 3. . Can K Form A Negative Ion.
From slideplayer.com
Ions Continued Unit 3 Topic ppt download Can K Form A Negative Ion if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. remember that ions are formed only when electrons move from one atom to another; A proton never moves from. Can K Form A Negative Ion.
From quizanisotropy.z4.web.core.windows.net
Do Metals Form Positive Or Negative Ions Can K Form A Negative Ion A proton never moves from one atom to another. remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must gain three electrons to have the same number of electrons as an. Naming ions and ionic compounds. nonmetals form negative ions (anions). if q 1 and q 2 have opposite. Can K Form A Negative Ion.
From exodvnood.blob.core.windows.net
Can Neon Form A Negative Ion at Zaida Wright blog Can K Form A Negative Ion High school chemistry > unit 3. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another.. Can K Form A Negative Ion.
From learningschoollavad0s.z14.web.core.windows.net
Positive Ions And Negative Ions Table Can K Form A Negative Ion nonmetals form negative ions (anions). remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl. Can K Form A Negative Ion.
From www.wou.edu
CH104 Chapter 3 Ions and Ionic Compounds Chemistry Can K Form A Negative Ion A proton never moves from one atom to. A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Naming ions and ionic compounds. nonmetals form negative ions (anions).. Can K Form A Negative Ion.
From www.pinterest.com
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry Can K Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. A proton never moves from one atom to. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. a cation (a positive ion) forms when a neutral atom. Can K Form A Negative Ion.
From dxogyodiq.blob.core.windows.net
Bromine Is Anion Or Cation at Glenn Kirby blog Can K Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. A proton never moves from one atom to another. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative,. Can K Form A Negative Ion.
From printablestojanir.z22.web.core.windows.net
Chemistry Positive And Negative Ions Can K Form A Negative Ion A proton never moves from one atom to. Naming ions and ionic compounds. nonmetals form negative ions (anions). a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. a cation (a positive ion) forms when a neutral atom loses one or more electrons. Can K Form A Negative Ion.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Can K Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Can K Form A Negative Ion.
From www.tes.com
AQA ion tests revision notes bundle, positive and negative ions Can K Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. A proton never moves from one atom to another. Naming ions and ionic compounds.. Can K Form A Negative Ion.
From www.tes.com
Polyatomic Ions Lessons TES Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. A proton never moves from one atom to. nonmetals form negative ions (anions). Naming ions and ionic compounds. remember that ions are formed only when electrons move from one atom to another; A. Can K Form A Negative Ion.
From tiklozi.weebly.com
Negative ions tiklozi Can K Form A Negative Ion A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. High school chemistry > unit 3. A proton never moves from one atom to.. Can K Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Can K Form A Negative Ion nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an. remember that ions are formed only when electrons move from one atom to another; remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when. Can K Form A Negative Ion.
From printableschooltackett.z21.web.core.windows.net
Chemistry Positive And Negative Ions Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl. Can K Form A Negative Ion.
From exodvnood.blob.core.windows.net
Can Neon Form A Negative Ion at Zaida Wright blog Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. nonmetals form negative ions (anions). Naming ions and ionic compounds. remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must. Can K Form A Negative Ion.
From slideplayer.com
Naming Ionic Compounds ppt download Can K Form A Negative Ion High school chemistry > unit 3. remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another. remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when a neutral atom loses one or more. Can K Form A Negative Ion.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Printable Form, Templates Can K Form A Negative Ion nonmetals form negative ions (anions). Naming ions and ionic compounds. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Can K Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Can K Form A Negative Ion A proton never moves from one atom to another. nonmetals form negative ions (anions). High school chemistry > unit 3. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy. Can K Form A Negative Ion.
From slideplayer.com
Compounds, Atoms, and Ions ppt download Can K Form A Negative Ion Naming ions and ionic compounds. A proton never moves from one atom to another. nonmetals form negative ions (anions). remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a. Can K Form A Negative Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. remember that ions are formed only when electrons move from one atom to another; High school chemistry > unit 3. nonmetals form negative ions (anions). A nitrogen atom. Can K Form A Negative Ion.
From slideplayer.com
Chapter 8 Chemical Bonding ppt download Can K Form A Negative Ion remember that ions are formed only when electrons move from one atom to another; remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral.. Can K Form A Negative Ion.
From schematicdiagramyakuza.z13.web.core.windows.net
Lewis Dot Symbols For All Elements Can K Form A Negative Ion remember that ions are formed only when electrons move from one atom to another; nonmetals form negative ions (anions). A proton never moves from one atom to another. Naming ions and ionic compounds. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative.. Can K Form A Negative Ion.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Can K Form A Negative Ion remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to. High school chemistry > unit 3. Naming ions and ionic compounds. nonmetals form negative ions (anions). A proton never moves from one atom to another. a cation (a positive ion) forms when a neutral atom. Can K Form A Negative Ion.
From slidetodoc.com
Crystal Binding Bonding Part IV Quantitative Models of Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral. remember that ions are formed only when electrons move from one atom to another; a cation (a positive ion) forms when a neutral atom loses one or more. Can K Form A Negative Ion.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6.2 Common Ions Can K Form A Negative Ion if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. remember that ions are formed only when electrons move from one atom to another; A nitrogen atom must gain. Can K Form A Negative Ion.
From schematicdiagramyakuza.z13.web.core.windows.net
Diagram Of Ionic Compound Can K Form A Negative Ion High school chemistry > unit 3. A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. remember that ions are formed only when electrons move from one atom. Can K Form A Negative Ion.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Can K Form A Negative Ion A proton never moves from one atom to another. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. A proton never moves from one atom to. A nitrogen atom. Can K Form A Negative Ion.
From ar.inspiredpencil.com
Ion Symbol Chart Can K Form A Negative Ion if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell,. Can K Form A Negative Ion.
From www.chemicals.co.uk
What Is An Ion In Chemistry? The Science Blog Can K Form A Negative Ion A proton never moves from one atom to. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. A nitrogen atom must gain three electrons to have the same number. Can K Form A Negative Ion.
From www.nagwa.com
Question Video Identifying Which Element Can Form a Positive Ion in an Can K Form A Negative Ion A proton never moves from one atom to. A proton never moves from one atom to another. if q 1 and q 2 have opposite signs (as in nacl, for example, where q 1 is +1 for na + and q 2 is −1 for cl −), then e is negative, which means that energy is. A nitrogen atom. Can K Form A Negative Ion.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Can K Form A Negative Ion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. nonmetals form negative ions (anions). a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a. Can K Form A Negative Ion.
From ionspecphilippines.com
Negative Ion IONSPEC Can K Form A Negative Ion nonmetals form negative ions (anions). remember that ions are formed only when electrons move from one atom to another; Naming ions and ionic compounds. A proton never moves from one atom to another. High school chemistry > unit 3. A nitrogen atom must gain three electrons to have the same number of electrons as an. a cation. Can K Form A Negative Ion.