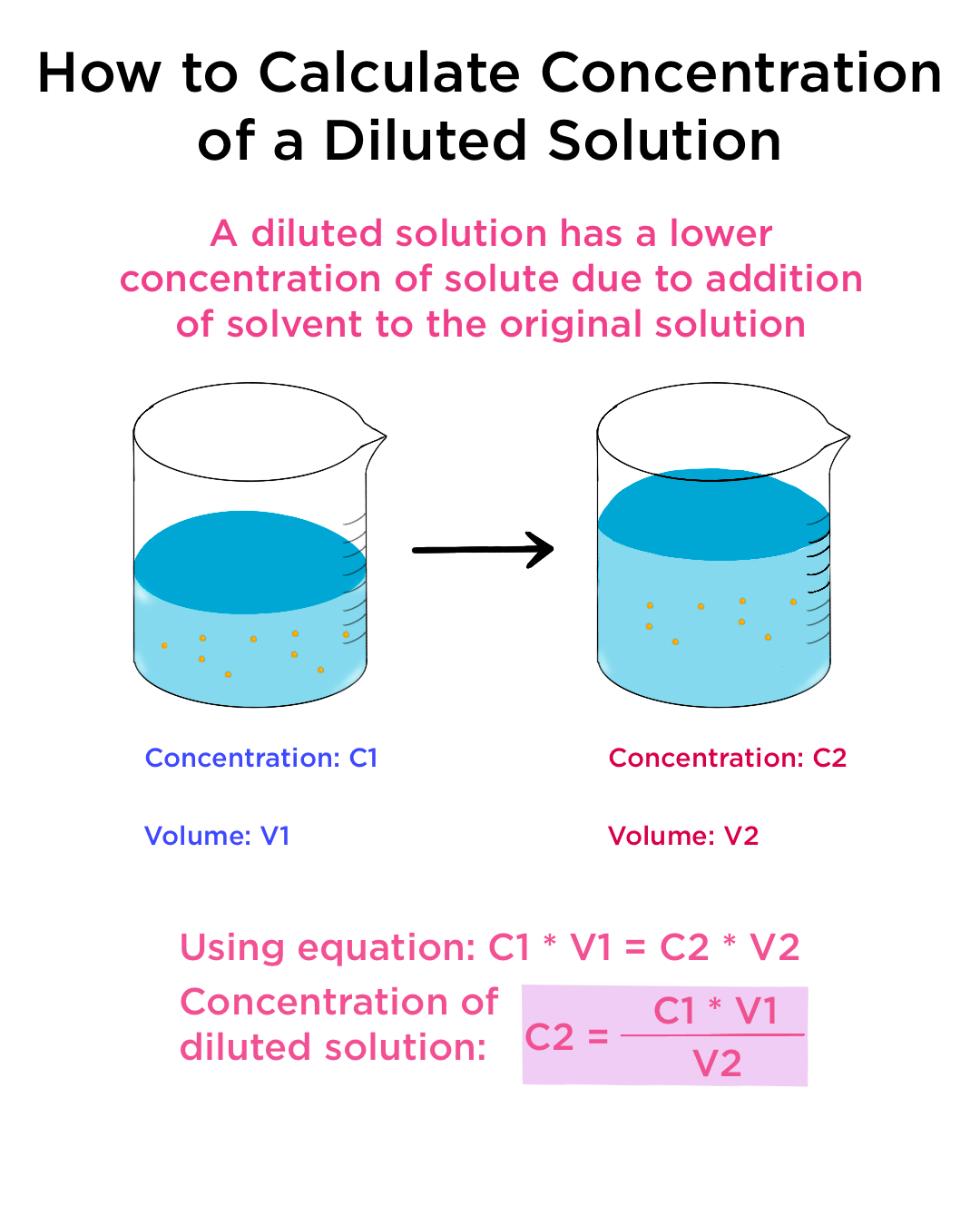
Dilution And Concentration Units . In both dilution and concentration,. The relative amount of a given solute in a solution is known as its concentration. Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. Concentration is the removal of solvent, which increases the concentration of. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions.
from www.expii.com
Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which increases the concentration of. Often, a worker will need to change the concentration of a solution by changing the. The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of.
Dilution of Solutions — Overview & Examples Expii
Dilution And Concentration Units Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. In both dilution and concentration,. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions.
From www.pinterest.com
Units of Concentration Should've probably printed this for Analytical lab, haha Chemistry Dilution And Concentration Units Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation, free download ID543259 Dilution And Concentration Units Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Concentration is the removal of solvent,. Dilution And Concentration Units.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download ID1709572 Dilution And Concentration Units In both dilution and concentration,. The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of. State whether the concentration. Dilution And Concentration Units.
From thecontentauthority.com
Dilution vs Concentration Deciding Between Similar Terms Dilution And Concentration Units The relative amount of a given solute in a solution is known as its concentration. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. In both dilution and concentration,. State whether the. Dilution And Concentration Units.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation, free download ID4108024 Dilution And Concentration Units Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. Dilution is the. Dilution And Concentration Units.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and chemistry YouTube Dilution And Concentration Units Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Concentration is the removal of solvent, which increases the concentration of. In both dilution and concentration,. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker. Dilution And Concentration Units.
From www.scribd.com
Molarity and Dilutions PDF Concentration Mole (Unit) Dilution And Concentration Units Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Often, a. Dilution And Concentration Units.
From www.slideshare.net
Molarity and dilution Dilution And Concentration Units Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. In both dilution and concentration,. Often, a worker will need to change the concentration of a solution by. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation, free download ID543259 Dilution And Concentration Units Learn how to dilute and concentrate solutions. In both dilution and concentration,. Dilution is the addition of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilutions and concentrations Lab 7 PowerPoint Presentation, free download ID9509568 Dilution And Concentration Units Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Learn how to dilute and concentrate solutions. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly. Dilution And Concentration Units.
From www.slideserve.com
PPT Concentrations of Solutions PowerPoint Presentation, free download ID5768427 Dilution And Concentration Units Dilution is the addition of. Learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which increases the concentration of. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation, free download ID543259 Dilution And Concentration Units Often, a worker will need to change the concentration of a solution by changing the. The relative amount of a given solute in a solution is known as its concentration. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing. Dilution And Concentration Units.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution And Concentration Units The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of. Learn how to dilute and concentrate solutions. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker. Dilution And Concentration Units.
From www.youtube.com
Dilution and Concentration Calculations in Pharmacy 5 Key Examples Solved YouTube Dilution And Concentration Units Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. Dilution And Concentration Units.
From www.youtube.com
Chem 11 Unit 5 Dilution Calculations Solving for Final Concentration YouTube Dilution And Concentration Units In both dilution and concentration,. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how. Dilution And Concentration Units.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution And Concentration Units State whether the concentration of a solution is directly or indirectly proportional to its volume. The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the. In both dilution and concentration,. Learn how to dilute and. Dilution And Concentration Units.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution And Concentration Units Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions.. Dilution And Concentration Units.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 Dilution And Concentration Units Concentration is the removal of solvent, which increases the concentration of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The relative amount of a given solute in a solution is known as its concentration. Often, a worker will need to change the concentration of a solution by changing the. Dilution. Dilution And Concentration Units.
From www.youtube.com
SCH3U1 / UNIT 3 / Solution Concentration and Dilutions YouTube Dilution And Concentration Units Concentration is the removal of solvent, which increases the concentration of. Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Solute. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation ID543259 Dilution And Concentration Units Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases. Dilution And Concentration Units.
From differencebtw.com
Dilution vs. Concentration Know the Difference Dilution And Concentration Units Dilution is the addition of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. Concentration is the removal of solvent, which increases the concentration of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to. Dilution And Concentration Units.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation, free download ID543259 Dilution And Concentration Units Learn how to dilute and concentrate solutions. In both dilution and concentration,. Learn how to dilute and concentrate solutions. The relative amount of a given solute in a solution is known as its concentration. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. State whether the. Dilution And Concentration Units.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Formula & Equation Stock Dilution And Concentration Units Learn how to dilute and concentrate solutions. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. The relative amount of a given solute in a solution is known as its concentration. In both dilution and concentration,. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing. Dilution And Concentration Units.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution And Concentration Units Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. The relative amount of a given solute in a solution is known as its concentration. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which. Dilution And Concentration Units.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Dilution YouTube Dilution And Concentration Units Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of. Dilution. Dilution And Concentration Units.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation, free download ID4108024 Dilution And Concentration Units In both dilution and concentration,. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Often, a worker will need to change the concentration of a solution by changing the.. Dilution And Concentration Units.
From www.scribd.com
How To Make Simple Solutions and Dilutions Unit Definitions PDF Concentration Molar Dilution And Concentration Units Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to. Dilution And Concentration Units.
From www.scribd.com
Understanding Concentration Units, Calculating Dilutions, and Expressing the Amount of Solute in Dilution And Concentration Units Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration,. The relative amount of a given solute in a solution is known as its concentration. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Dilution is the. Dilution And Concentration Units.
From www.youtube.com
How to Solve Concentration and Dilutions Formula! PTCB Calculations YouTube Dilution And Concentration Units The relative amount of a given solute in a solution is known as its concentration. Concentration is the removal of solvent, which increases the concentration of. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Learn how to dilute and concentrate solutions. Dilution is the addition of. Often, a worker will need to change the. Dilution And Concentration Units.
From www.youtube.com
How to calculate dilution and concentration YouTube Dilution And Concentration Units State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of solvent, which decreases the concentration of. Dilution And Concentration Units.
From www.researchgate.net
Top 5 substances concentration and threshold dilution of relevant unit. Download Scientific Dilution And Concentration Units State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. Concentration is the removal of solvent, which increases the concentration of. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. The relative amount of a given solute in a solution is known as its concentration.. Dilution And Concentration Units.
From www.scribd.com
Solution Preparation and Dilutions PDF Molar Concentration Mole (Unit) Dilution And Concentration Units Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the concentration of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. The relative amount of a given solute in a solution is known as its concentration.. Dilution And Concentration Units.
From www.scribd.com
Molarity Molality and Dilutions PDF Concentration Mole (Unit) Dilution And Concentration Units Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution is the addition of. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. The relative amount of a given solute in a solution is known as its concentration. In both. Dilution And Concentration Units.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution And Concentration Units The relative amount of a given solute in a solution is known as its concentration. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the addition of. Learn how to dilute and concentrate. Dilution And Concentration Units.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution And Concentration Units Concentration is the removal of solvent, which increases the concentration of. In both dilution and concentration,. State whether the concentration of a solution is directly or indirectly proportional to its volume. Solute concentrations are often described with qualitative terms such as dilute (of relatively low. Often, a worker will need to change the concentration of a solution by changing the.. Dilution And Concentration Units.