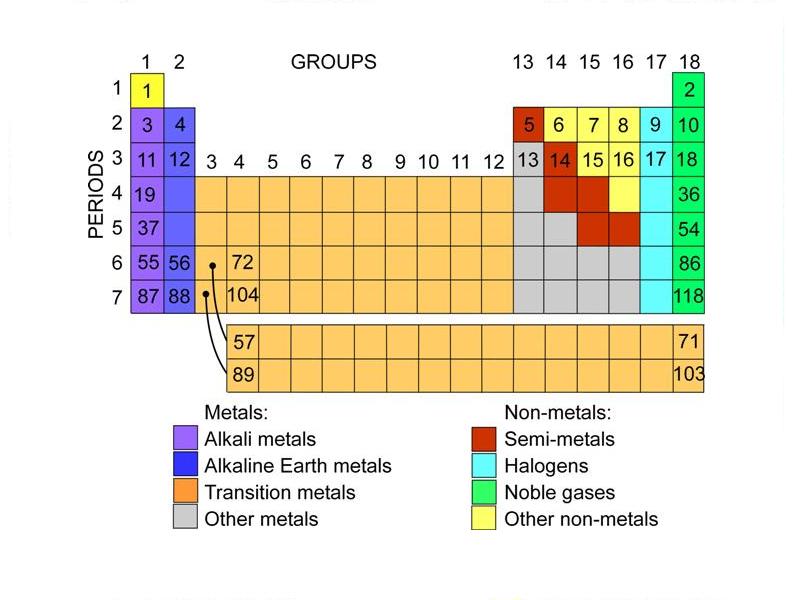
Which Group 14 Element Is A Metalloid . A quartz crystal is a macromolecule of silicon dioxide. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Carbon is followed by silicon (si) and germanium. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but has at least one.
from www.sciencelearn.org.nz
Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but has at least one. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The difference between these two compounds is the ability of the group 14. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A quartz crystal is a macromolecule of silicon dioxide. Carbon is followed by silicon (si) and germanium. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group.
Periodic table of elements — Science Learning Hub
Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The difference between these two compounds is the ability of the group 14. A quartz crystal is a macromolecule of silicon dioxide. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Each metalloid element takes many forms, but has at least one. Carbon is followed by silicon (si) and germanium. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy.
From utedzz.blogspot.com
Periodic Table Metalloids Periodic Table Timeline Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are elements with properties intermediate. Which Group 14 Element Is A Metalloid.
From www.slideserve.com
PPT The Periodic Table of the Elements PowerPoint Presentation, free download ID2966028 Which Group 14 Element Is A Metalloid Carbon is followed by silicon (si) and germanium. The difference between these two compounds is the ability of the group 14. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Each metalloid element. Which Group 14 Element Is A Metalloid.
From www.haikudeck.com
Metalloids by Megan Maul Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. The difference between these two compounds. Which Group 14 Element Is A Metalloid.
From utedzz.blogspot.com
Periodic Table Group 14 Periodic Table Timeline Which Group 14 Element Is A Metalloid The difference between these two compounds is the ability of the group 14. A quartz crystal is a macromolecule of silicon dioxide. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group 14 of the periodic table. Which Group 14 Element Is A Metalloid.
From slidetodoc.com
Properties of the Periodic Table The Periodic Table Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Carbon is followed by silicon (si) and germanium. Each metalloid element takes many forms, but has at least one.. Which Group 14 Element Is A Metalloid.
From sciencenotes.org
List of Metalloids or Semimetals Which Group 14 Element Is A Metalloid The difference between these two compounds is the ability of the group 14. Carbon is followed by silicon (si) and germanium. A quartz crystal is a macromolecule of silicon dioxide. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but has at least one. A. Which Group 14 Element Is A Metalloid.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. A quartz crystal is a macromolecule of silicon dioxide. Carbon is followed by silicon (si) and germanium. The metalloids or semimetals are elements with properties. Which Group 14 Element Is A Metalloid.
From quizlet.com
Periodic Table of Elements with Group Names Diagram Quizlet Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. A quartz crystal is a macromolecule of silicon dioxide. Group 14 elements are less electropositive than group 13 owing to their small size and high. Which Group 14 Element Is A Metalloid.
From www.pathwaystochemistry.com
Common Groups of Elements Pathways to Chemistry Which Group 14 Element Is A Metalloid Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Carbon is followed by silicon (si) and germanium. A series of six elements called the metalloids separate. Which Group 14 Element Is A Metalloid.
From www.ck12.org
Electron Configuration and the Periodic Table CK12 Foundation Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A quartz crystal is a macromolecule of silicon dioxide. Carbon is followed by silicon (si) and germanium. The difference between these two compounds is the ability of the group 14. Group 14 elements are less electropositive than group 13 owing to their small size and high. Which Group 14 Element Is A Metalloid.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Which Group 14 Element Is A Metalloid Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Carbon is followed by silicon (si) and germanium. The difference between these two compounds is the ability of the group 14. A series of six elements called the. Which Group 14 Element Is A Metalloid.
From www.xometry.com
7 Elements of Metalloids Differences and Uses Xometry Which Group 14 Element Is A Metalloid Carbon is followed by silicon (si) and germanium. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The difference between these two compounds is the ability of the group 14. Each metalloid element takes many forms, but has at least one. A series of six elements called the metalloids separate the metals from the nonmetals. Which Group 14 Element Is A Metalloid.
From courses.lumenlearning.com
Occurrence, Preparation, and Properties of Transition Metals and Their Compounds Chemistry for Which Group 14 Element Is A Metalloid A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but has at least one. A quartz crystal is a macromolecule of silicon dioxide. The difference between these. Which Group 14 Element Is A Metalloid.
From www.freepik.com
Premium Vector Silicon is chemical element number 14 of the metalloid group Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Carbon is followed by silicon (si) and germanium. A quartz crystal is a macromolecule of silicon dioxide. The difference between these two compounds is the ability of the group 14. Each metalloid element takes many forms, but has at least one. A series of six elements. Which Group 14 Element Is A Metalloid.
From www.breakingatom.com
Metalloid Definition Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. Carbon is followed by silicon (si) and germanium. Each metalloid element takes many forms, but has at least one. The metalloids or semimetals are elements. Which Group 14 Element Is A Metalloid.
From mungfali.com
Metals Nonmetals And Metalloids Chart Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. The difference between these two compounds is the ability of the group 14. A quartz crystal is a macromolecule of silicon dioxide. A series of six elements called. Which Group 14 Element Is A Metalloid.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. The difference between these two compounds is the ability of the group 14. Carbon is followed by silicon (si) and germanium. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. A series of six elements called the metalloids. Which Group 14 Element Is A Metalloid.
From www.sciencelearn.org.nz
Periodic table of elements — Science Learning Hub Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. Carbon is followed by silicon (si) and germanium. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Group 14 of the periodic table is. Which Group 14 Element Is A Metalloid.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. The difference between these two compounds is the ability of the group 14. Carbon is followed by silicon (si) and germanium. Each metalloid element takes many forms, but has at least one. A. Which Group 14 Element Is A Metalloid.
From brokeasshome.com
Metalloids Located On The Periodic Table Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The difference between these two compounds. Which Group 14 Element Is A Metalloid.
From www.ck12.org
Groups of Elements CK12 Foundation Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. Carbon is followed by silicon (si) and germanium. The difference between these two compounds is the ability of the group 14. Each metalloid element takes many forms, but has at least one. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group. Which Group 14 Element Is A Metalloid.
From www.thoughtco.com
List of Halogens (Element Groups) Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Carbon is followed by silicon (si) and germanium. Each metalloid element takes many forms, but has at least one. The difference between these two compounds is the ability of the group 14. Group 14 of the periodic table is headed by the nonmetal carbon (c), so. Which Group 14 Element Is A Metalloid.
From www.adda247.com
What are metalloids? Definition, Properties and Example Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Each metalloid element takes many forms, but. Which Group 14 Element Is A Metalloid.
From periodictable.me
Labeled Periodic Table of Elements with Name Dynamic Periodic Table of Elements and Chemistry Which Group 14 Element Is A Metalloid A quartz crystal is a macromolecule of silicon dioxide. Each metalloid element takes many forms, but has at least one. The difference between these two compounds is the ability of the group 14. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Group 14 elements are less electropositive than group 13 owing to their small. Which Group 14 Element Is A Metalloid.
From www.breakingatom.com
Periodic Table Metalloid Which Group 14 Element Is A Metalloid Each metalloid element takes many forms, but has at least one. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Carbon is followed by silicon (si) and germanium. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Group 14 of the periodic table is headed by. Which Group 14 Element Is A Metalloid.
From ar.inspiredpencil.com
Periodic Table Metals Nonmetals Metalloids Labeled Which Group 14 Element Is A Metalloid Carbon is followed by silicon (si) and germanium. Each metalloid element takes many forms, but has at least one. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The difference between these two compounds is the ability of the group 14. A series of six elements called. Which Group 14 Element Is A Metalloid.
From studylib.net
Group 14 Elements Which Group 14 Element Is A Metalloid Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Carbon is followed by silicon (si) and germanium. A quartz crystal is a macromolecule of silicon dioxide. Each metalloid element takes many forms, but. Which Group 14 Element Is A Metalloid.
From fphoto.photoshelter.com
science element carbon group 14 Fundamental Photographs The Art of Science Which Group 14 Element Is A Metalloid Carbon is followed by silicon (si) and germanium. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. The metalloids or semimetals are elements with properties intermediate between the metals. Which Group 14 Element Is A Metalloid.
From www.youtube.com
How to identify METALS NONMETALS METALLOIDS on the PERIODIC TABLE YouTube Which Group 14 Element Is A Metalloid The difference between these two compounds is the ability of the group 14. The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. Carbon is followed by silicon (si) and germanium. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. A series of six elements called the. Which Group 14 Element Is A Metalloid.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. The difference between these two compounds is the ability of the group 14. Each metalloid element takes many forms, but has at least one. A quartz crystal is a macromolecule of silicon dioxide. A series of six elements called the metalloids separate the metals from the. Which Group 14 Element Is A Metalloid.
From sciencenotes.org
Metalloids Science Notes and Projects Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. A quartz crystal is a macromolecule of silicon dioxide. Each metalloid element takes many forms, but has at least one. Carbon is followed by silicon (si) and germanium.. Which Group 14 Element Is A Metalloid.
From nigerianscholars.com
Periodicity Metals, Metalloids, and Nonmetals Which Group 14 Element Is A Metalloid The difference between these two compounds is the ability of the group 14. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. A quartz crystal is a macromolecule of silicon dioxide. Each metalloid element takes many forms, but has at least one. Carbon is followed by silicon (si) and germanium. The. Which Group 14 Element Is A Metalloid.
From www.periodictableprintable.com
Periodic Table Of Elements With Names Metals And Nonmetals Periodic Table Printable Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Group 14 of the periodic table is headed by the. Which Group 14 Element Is A Metalloid.
From www.slideshare.net
The Periodic Table Which Group 14 Element Is A Metalloid The metalloids or semimetals are elements with properties intermediate between the metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Each metalloid element takes many forms, but has at least one. A quartz crystal is a macromolecule of silicon dioxide. The difference between these two compounds is the. Which Group 14 Element Is A Metalloid.
From sciencenotes.org
Main Group Elements Definition and Importance Which Group 14 Element Is A Metalloid Carbon is followed by silicon (si) and germanium. A quartz crystal is a macromolecule of silicon dioxide. Group 14 elements are less electropositive than group 13 owing to their small size and high ionization enthalpy. Group 14 of the periodic table is headed by the nonmetal carbon (c), so this group is also called the carbon group. Each metalloid element. Which Group 14 Element Is A Metalloid.