What Does A Dilution Factor Of 100 Mean . The formula for dilution factor. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. The dilution factor is often used as the denominator of a fraction. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. What is a dilution factor and what does 1:50 mean? It may be expressed as the ratio of the volume of the final. You have diluted the sample by a factor of 100. The dilution factor would be: Dilution factor is the factor by which the stock solution is diluted. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution.
from www.slideserve.com
The dilution factor is often used as the denominator of a fraction. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. It may be expressed as the ratio of the volume of the final. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. You have diluted the sample by a factor of 100. What is a dilution factor and what does 1:50 mean? Dilution factor is the factor by which the stock solution is diluted. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution.
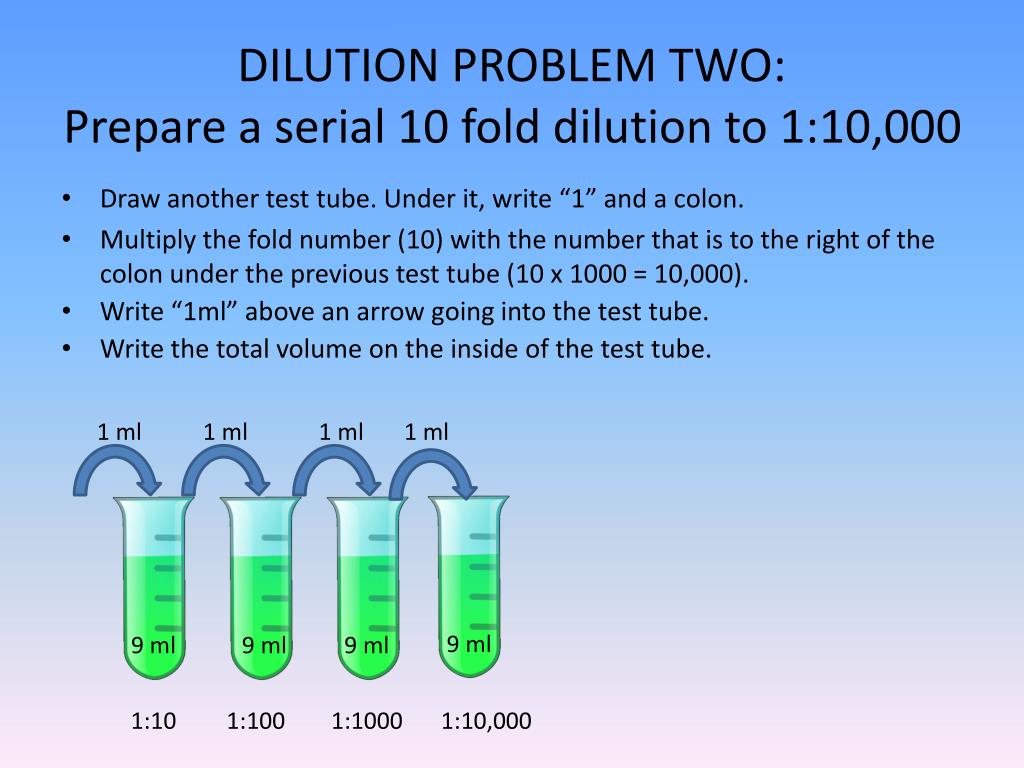
PPT Study Guide for Dilution PROBLEMS and Concentrations problems
What Does A Dilution Factor Of 100 Mean Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The dilution factor would be: Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The dilution factor is often used as the denominator of a fraction. The formula for dilution factor. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution factor is the factor by which the stock solution is diluted. It may be expressed as the ratio of the volume of the final. You have diluted the sample by a factor of 100. What is a dilution factor and what does 1:50 mean? Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory What Does A Dilution Factor Of 100 Mean For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The dilution factor is often used as the denominator of a fraction. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that. What Does A Dilution Factor Of 100 Mean.
From www.pdfprof.com
how to calculate dilution factor What Does A Dilution Factor Of 100 Mean The dilution factor would be: The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. The dilution factor is often used as the denominator of a fraction. The formula for dilution. What Does A Dilution Factor Of 100 Mean.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen What Does A Dilution Factor Of 100 Mean The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. What is a dilution factor and what does 1:50 mean? Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. Dilution factor refers to. What Does A Dilution Factor Of 100 Mean.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID What Does A Dilution Factor Of 100 Mean The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. It may be expressed as the ratio of the volume of the final. The dilution factor would be: Dilution factor is the factor by which the stock solution is diluted. What is a dilution factor and what does 1:50 mean? Dilution factor refers to the. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in What Does A Dilution Factor Of 100 Mean The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. It may be expressed as the ratio of the volume of the final. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1,. What Does A Dilution Factor Of 100 Mean.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems What Does A Dilution Factor Of 100 Mean The dilution factor would be: The formula for dilution factor. It may be expressed as the ratio of the volume of the final. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. For example, if 100 ml of a stock solution is diluted with. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
How to Calculate Dilution Factor YouTube What Does A Dilution Factor Of 100 Mean The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. What is a dilution factor and what does 1:50 mean? Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. For example, if 100 ml of a. What Does A Dilution Factor Of 100 Mean.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic What Does A Dilution Factor Of 100 Mean What is a dilution factor and what does 1:50 mean? The formula for dilution factor. Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1,. What Does A Dilution Factor Of 100 Mean.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory What Does A Dilution Factor Of 100 Mean The dilution factor would be: The dilution factor is often used as the denominator of a fraction. It may be expressed as the ratio of the volume of the final. You have diluted the sample by a factor of 100. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. What. What Does A Dilution Factor Of 100 Mean.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology What Does A Dilution Factor Of 100 Mean Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. You have diluted the sample by a factor of 100. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. The “1:50”. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube What Does A Dilution Factor Of 100 Mean Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. The formula for dilution factor. Dilution factor is the factor by which the stock solution is diluted. Dilution factor is a ratio or exponent that shows how much of the original solution is left after. What Does A Dilution Factor Of 100 Mean.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer What Does A Dilution Factor Of 100 Mean The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final. What Does A Dilution Factor Of 100 Mean.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions What Does A Dilution Factor Of 100 Mean You have diluted the sample by a factor of 100. What is a dilution factor and what does 1:50 mean? For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube What Does A Dilution Factor Of 100 Mean It may be expressed as the ratio of the volume of the final. The dilution factor would be: The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. The formula for dilution factor. What is a dilution factor and what does 1:50 mean? Dilution factor is the factor by which the stock solution is diluted.. What Does A Dilution Factor Of 100 Mean.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY What Does A Dilution Factor Of 100 Mean Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The formula for dilution factor. Dilution factor (df)=vi/ vf for instance, if. What Does A Dilution Factor Of 100 Mean.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer What Does A Dilution Factor Of 100 Mean The dilution factor would be: Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. The dilution factor is often used as the denominator of a fraction. The formula for dilution factor. You have diluted the sample by a factor of 100. It may be. What Does A Dilution Factor Of 100 Mean.
From lasopamail784.weebly.com
Calculate Dilution Factor lasopamail What Does A Dilution Factor Of 100 Mean Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. The formula for dilution factor. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. The dilution factor would be: The dilution factor. What Does A Dilution Factor Of 100 Mean.
From promolasopa510.weebly.com
Calculate dilution factor promolasopa What Does A Dilution Factor Of 100 Mean The formula for dilution factor. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. It may be expressed as the. What Does A Dilution Factor Of 100 Mean.
From www.medicine.mcgill.ca
Serial Dilutions What Does A Dilution Factor Of 100 Mean It may be expressed as the ratio of the volume of the final. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution factor (df)=vi/ vf. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
How Do You Calculate Dilution Factor? YouTube What Does A Dilution Factor Of 100 Mean The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The dilution factor would be: You have diluted the sample by a factor of 100. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The formula. What Does A Dilution Factor Of 100 Mean.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality What Does A Dilution Factor Of 100 Mean Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. You have diluted the sample by a factor of 100. It may be expressed as the ratio of the volume of the final. The dilution factor is often used as the denominator of a fraction. The formula for dilution factor. The. What Does A Dilution Factor Of 100 Mean.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query What Does A Dilution Factor Of 100 Mean What is a dilution factor and what does 1:50 mean? You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is diluted. It may be expressed as the ratio of the volume of the final. The dilution factor would be: Dilution factor is a ratio or exponent that shows how. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
Calculating Dilution Factor YouTube What Does A Dilution Factor Of 100 Mean The dilution factor is often used as the denominator of a fraction. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. What is a dilution factor and what does 1:50 mean? It may be expressed as the ratio of the volume of the final. The. What Does A Dilution Factor Of 100 Mean.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration What Does A Dilution Factor Of 100 Mean Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. What is a dilution factor and what does 1:50 mean? The formula for dilution factor. You have. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and What Does A Dilution Factor Of 100 Mean The dilution factor would be: The formula for dilution factor. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. What is a dilution factor and what does 1:50 mean? Dilution factor is the factor by. What Does A Dilution Factor Of 100 Mean.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems What Does A Dilution Factor Of 100 Mean Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. You have diluted the sample by a factor of 100. The formula for dilution factor. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. What. What Does A Dilution Factor Of 100 Mean.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii What Does A Dilution Factor Of 100 Mean Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. What is a dilution factor and what does 1:50 mean? For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml,. What Does A Dilution Factor Of 100 Mean.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems What Does A Dilution Factor Of 100 Mean You have diluted the sample by a factor of 100. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Dilution factor. What Does A Dilution Factor Of 100 Mean.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples What Does A Dilution Factor Of 100 Mean The dilution factor is often used as the denominator of a fraction. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution to the volume of the final (dilute) solution 1, that is, the ratio of v. What is a dilution factor and what does 1:50 mean? It may be expressed as the ratio of the. What Does A Dilution Factor Of 100 Mean.
From www.omnicalculator.com
Dilution Factor Calculator What Does A Dilution Factor Of 100 Mean Dilution factor is the factor by which the stock solution is diluted. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. The formula for dilution factor. It may be expressed as the ratio of the volume of the final. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml. What Does A Dilution Factor Of 100 Mean.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate What Does A Dilution Factor Of 100 Mean The formula for dilution factor. It may be expressed as the ratio of the volume of the final. Dilution factor (df)=vi/ vf for instance, if you add 0.1 ml of a specimen to 9.9 ml of diluent, the final volume is 10 ml. What is a dilution factor and what does 1:50 mean? The dilution factor (or dilution ratio) is. What Does A Dilution Factor Of 100 Mean.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript What Does A Dilution Factor Of 100 Mean Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. Dilution factor is the factor by which the stock solution is diluted. The formula for dilution factor. You have diluted the sample by a factor of 100. Dilution factor refers to the ratio of the volume of the initial (concentrated) solution. What Does A Dilution Factor Of 100 Mean.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY What Does A Dilution Factor Of 100 Mean For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. The “1:50” indicates the dilution factor, or volume ratio, to utilise for making the new. The formula for dilution factor. The dilution factor (or dilution ratio) is the notation used to express how much of the. What Does A Dilution Factor Of 100 Mean.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology What Does A Dilution Factor Of 100 Mean The formula for dilution factor. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. Dilution factor is the factor by which the stock solution is diluted.. What Does A Dilution Factor Of 100 Mean.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free What Does A Dilution Factor Of 100 Mean For example, if 100 ml of a stock solution is diluted with solvent/diluent to a total, final volume of 1000 ml, the resulting dilution. You have diluted the sample by a factor of 100. Dilution factor is a ratio or exponent that shows how much of the original solution is left after dilution. Dilution factor refers to the ratio of. What Does A Dilution Factor Of 100 Mean.