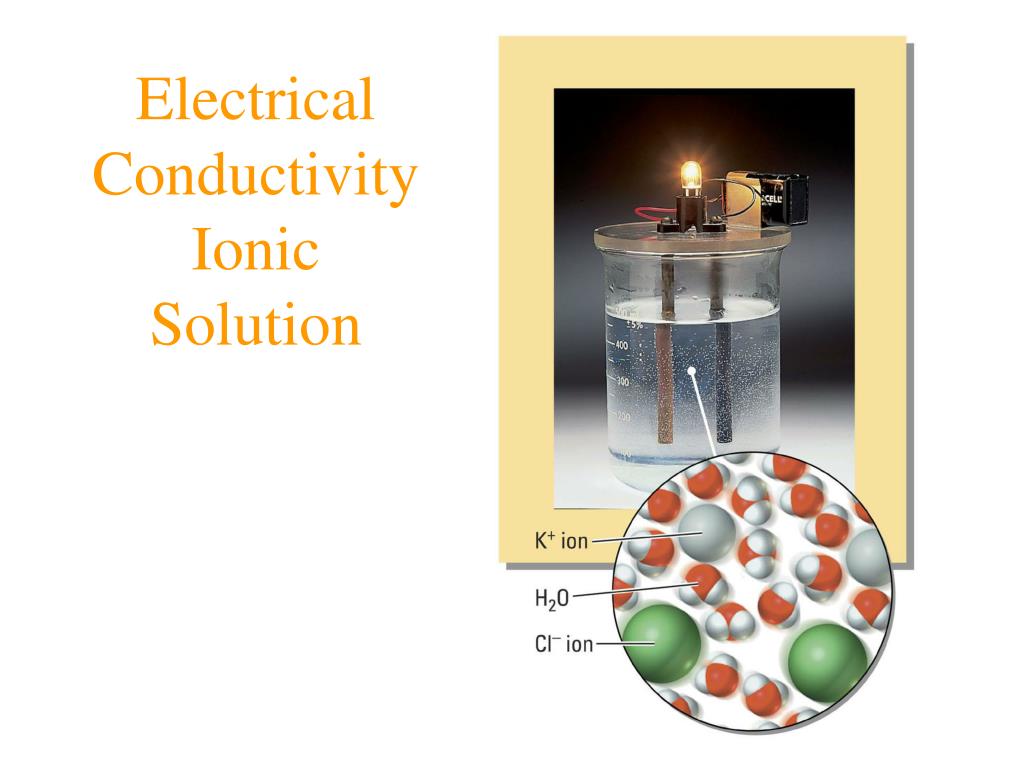
Are Ionic Compounds Conductive . In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). An ionic compound can be identified by its chemical formula: Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds conduct an electric current when melted or dissolved in water. Find out how ionic compounds conduct electricity when molten or dissolved in water. Metal + nonmetal or polyatomic ions. In an ionic solution, the. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Learn the difference between ionic and covalent bonds, and how ions.
from www.slideserve.com
Ionic compounds conduct an electric current when melted or dissolved in water. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Metal + nonmetal or polyatomic ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. An ionic compound can be identified by its chemical formula: Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Find out how ionic compounds conduct electricity when molten or dissolved in water.
PPT Compounds PowerPoint Presentation, free download ID6710250
Are Ionic Compounds Conductive Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Find out how ionic compounds conduct electricity when molten or dissolved in water. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. An ionic compound can be identified by its chemical formula: Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Learn the difference between ionic and covalent bonds, and how ions. Metal + nonmetal or polyatomic ions. In an ionic solution, the. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). An ionic compound can be identified by its chemical formula: Once dissolved or melted, ionic compounds are excellent conductors. Are Ionic Compounds Conductive.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Are Ionic Compounds Conductive Find out how ionic compounds conduct electricity when molten or dissolved in water. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are compounds that result from the electrical attraction. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID6710250 Are Ionic Compounds Conductive Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. In an ionic solution, the. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles).. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. In an ionic solution, the. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. An ionic compound can be identified by its chemical formula: Once. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2440715 Are Ionic Compounds Conductive Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Learn the difference between ionic and covalent bonds, and how ions. Ionic compounds conduct electricity in water because they dissociate into charged ions. Are Ionic Compounds Conductive.
From getchemistry.weebly.com
Ionic Bonding Chemistry Are Ionic Compounds Conductive Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Ionic compounds conduct an electric current when melted or dissolved in water. In solid form, an ionic compound is not electrically conductive because its ions. Are Ionic Compounds Conductive.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Are Ionic Compounds Conductive In an ionic solution, the. Learn the difference between ionic and covalent bonds, and how ions. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. An ionic compound can be identified by its chemical formula: Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent. Are Ionic Compounds Conductive.
From www.slideshare.net
Lecture 7.2 Ionic Compounds Are Ionic Compounds Conductive In an ionic solution, the. Find out how ionic compounds conduct electricity when molten or dissolved in water. An ionic compound can be identified by its chemical formula: In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds are compounds that result from the. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT IONIc bonds and ionic compounds PowerPoint Presentation ID2276705 Are Ionic Compounds Conductive In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Metal + nonmetal or polyatomic ions. Learn about the characteristics of ionic compounds, such as crystalline. Are Ionic Compounds Conductive.
From www.slideshare.net
Chem matters ch6_ionic_bond Are Ionic Compounds Conductive Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. An ionic compound can be identified by its chemical formula: Find out how ionic compounds conduct electricity when molten or dissolved in water. Ionic compounds conduct electricity in. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2440715 Are Ionic Compounds Conductive Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Find out how ionic. Are Ionic Compounds Conductive.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Are Ionic Compounds Conductive Learn the difference between ionic and covalent bonds, and how ions. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Solutions of ionic compounds and melted ionic. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Are Ionic Compounds Conductive An ionic compound can be identified by its chemical formula: In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct an electric current. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Are Ionic Compounds Conductive Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. In an ionic solution, the. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds. Are Ionic Compounds Conductive.
From www.youtube.com
Explaining the conductivity of ionic compounds YouTube Are Ionic Compounds Conductive Find out how ionic compounds conduct electricity when molten or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. An ionic compound can be identified by its chemical formula: Metal + nonmetal or polyatomic ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Are Ionic Compounds Conductive An ionic compound can be identified by its chemical formula: In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Find out how ionic compounds conduct electricity when molten or dissolved in water. Ionic compounds conduct an electric current when melted or dissolved in water. Solutions. Are Ionic Compounds Conductive.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary Are Ionic Compounds Conductive Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Metal + nonmetal or polyatomic ions. Find out how ionic compounds conduct electricity when molten or dissolved in water. Ionic compounds conduct electricity in water because. Are Ionic Compounds Conductive.
From www.smartexamresources.com
IGCSE Notes Ions And Ionic Bonds Smart Exam Resources Are Ionic Compounds Conductive Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. In. Are Ionic Compounds Conductive.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.56C Understand Why Ionic Compounds Conduct Are Ionic Compounds Conductive Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Metal + nonmetal or polyatomic ions. Ionic compounds conduct an electric current when melted or dissolved in water. Learn the difference between ionic and covalent. Are Ionic Compounds Conductive.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Are Ionic Compounds Conductive In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating. Are Ionic Compounds Conductive.
From www.youtube.com
Chemical Bonding (Electrical Conductivity of Ionic Compound) Concept Are Ionic Compounds Conductive Learn the difference between ionic and covalent bonds, and how ions. Metal + nonmetal or polyatomic ions. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. In an ionic solution, the. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Ionic compounds conduct an electric current when melted or. Are Ionic Compounds Conductive.
From www.coursehero.com
[Solved] ) 7. Which is a property of ionic compounds? O conductive when Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. In an ionic solution, the. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about. Are Ionic Compounds Conductive.
From www.youtube.com
How Ionic Compounds Conduct Electricity GCSE Chemistry kayscience Are Ionic Compounds Conductive Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. In an ionic solution, the. Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Once dissolved or melted, ionic compounds. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Ionic Bonds and Properties of Ionic Compounds PowerPoint Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. In an ionic solution, the. An ionic compound can be identified by its chemical formula: Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. Learn the difference between ionic and covalent bonds, and how ions. An ionic compound can be identified by its chemical formula: Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds conduct electricity in water because they dissociate into charged ions. Are Ionic Compounds Conductive.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Are Ionic Compounds Conductive Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Solutions of ionic compounds. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Covalent and Ionic Bonds PowerPoint Presentation, free download Are Ionic Compounds Conductive Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Metal + nonmetal or polyatomic ions. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Ionic compounds conduct an electric current when melted or dissolved in water. In solid form, an ionic compound is not electrically conductive because. Are Ionic Compounds Conductive.
From slideplayer.com
Structure and Properties of Bonds ppt download Are Ionic Compounds Conductive An ionic compound can be identified by its chemical formula: Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Metal + nonmetal or polyatomic ions. Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. In an ionic solution, the. Find out how ionic compounds conduct electricity. Are Ionic Compounds Conductive.
From studylib.net
Lab Conductivity of Ionic and Covalent Compounds Are Ionic Compounds Conductive An ionic compound can be identified by its chemical formula: Learn the difference between ionic and covalent bonds, and how ions. Metal + nonmetal or polyatomic ions. Find out how ionic compounds conduct electricity when molten or dissolved in water. Ionic compounds are compounds that result from the electrical attraction between oppositely charged ions. Ionic compounds conduct an electric current. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Ionic Conductivity and Solid Electrolytes I The Basics Are Ionic Compounds Conductive Find out how ionic compounds conduct electricity when molten or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. An ionic compound can be identified by its chemical. Are Ionic Compounds Conductive.
From philschatz.com
Electrolytes · Chemistry Are Ionic Compounds Conductive Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. In an ionic solution, the. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds conduct electricity in water because they dissociate into charged. Are Ionic Compounds Conductive.
From www.slideserve.com
PPT Ionic Compound Properties, Lewis Dot Structures & Polyatomics Are Ionic Compounds Conductive Learn the difference between ionic and covalent bonds, and how ions. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds conduct an electric current when melted or dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Are Ionic Compounds Conductive.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Are Ionic Compounds Conductive Metal + nonmetal or polyatomic ions. In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Learn about the characteristics of ionic compounds, such as crystalline solids, high melting points, and insulating solids. Find out how ionic compounds conduct electricity when molten or dissolved in water.. Are Ionic Compounds Conductive.
From slideplayer.com
Explaining the Physical Properties of Ionic Substances ppt download Are Ionic Compounds Conductive Ionic compounds conduct an electric current when melted or dissolved in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely. Learn the difference between ionic and covalent bonds, and how ions. Metal + nonmetal or polyatomic ions. In an ionic solution, the. In solid form, an ionic compound. Are Ionic Compounds Conductive.
From www.researchgate.net
The ionic conductivity of liquid electrolytes with various Are Ionic Compounds Conductive In an ionic solution, the. Learn the difference between ionic and covalent bonds, and how ions. Ionic compounds conduct an electric current when melted or dissolved in water. Ionic compounds conduct electricity in water because they dissociate into charged ions that can move in the solution. Find out how ionic compounds conduct electricity when molten or dissolved in water. Once. Are Ionic Compounds Conductive.