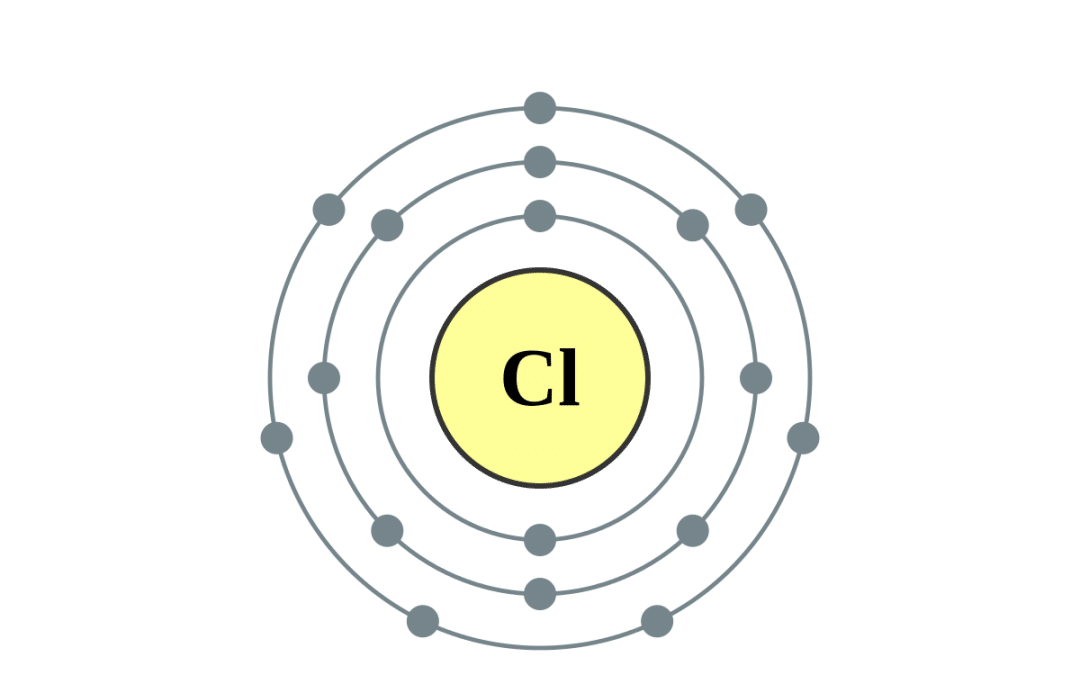
Chlorine Cl Electron Configuration . It is an extremely reactive element and a strong oxidising agent: Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Therefore, the ground state electron configuration of the element, chlorine is: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. It has 17 electrons, filling up to the 3p orbital. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Electron configuration of chlorine is [ne] 3s2 3p5. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as.
from chemtech-us.com
It is an extremely reactive element and a strong oxidising agent: It has 17 electrons, filling up to the 3p orbital. We'll need to know how many sublevel is present. Therefore, the ground state electron configuration of the element, chlorine is: How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Electron configuration chart of all elements is mentioned in the table below.
15 Interesting Facts About Chlorine
Chlorine Cl Electron Configuration The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Therefore, the ground state electron configuration of the element, chlorine is: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. The shorthand electron configuration (or noble gas configuration) as well as. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It has 17 electrons, filling up to the 3p orbital. We'll need to know how many sublevel is present. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. It is an extremely reactive element and a strong oxidising agent: Electron configuration chart of all elements is mentioned in the table below.
From sciencenotes.org
Chlorine Facts Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. Electron configuration chart of all elements is mentioned in the table below. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. The shorthand electron configuration (or noble gas configuration) as well as. Therefore,. Chlorine Cl Electron Configuration.
From www.dreamstime.com
Diagram Representation of the Element Chlorine Stock Illustration Chlorine Cl Electron Configuration How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. We'll need to know how many sublevel is present. It has 17 electrons, filling up to the 3p orbital. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17. Chlorine Cl Electron Configuration.
From imgbin.com
Electron Configuration Aufbau Principle Valence Electron Chlorine PNG Chlorine Cl Electron Configuration It is an extremely reactive element and a strong oxidising agent: Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron. Chlorine Cl Electron Configuration.
From mavink.com
Aufbau Diagram For Chlorine Chlorine Cl Electron Configuration #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. It is an extremely reactive element and a strong oxidising agent: How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Electron configuration of chlorine is [ne] 3s2 3p5. The shorthand electron configuration (or noble gas configuration) as well. Chlorine Cl Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Cl Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. We'll need to know how many sublevel is present. Chlorine is a chemical element with atomic number 17 which means. Chlorine Cl Electron Configuration.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Cl Electron Configuration Electron configuration of chlorine is [ne] 3s2 3p5. The shorthand electron configuration (or noble gas configuration) as well as. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Therefore, the ground state electron configuration of the element, chlorine is: We'll need to know how many. Chlorine Cl Electron Configuration.
From galvinconanstuart.blogspot.com
Atomic Orbital Diagram For Chlorine General Wiring Diagram Chlorine Cl Electron Configuration We'll need to know how many sublevel is present. Therefore, the ground state electron configuration of the element, chlorine is: It has 17 electrons, filling up to the 3p orbital. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Chlorine is. Chlorine Cl Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Cl Electron Configuration #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. It is an extremely reactive element and a strong oxidising agent: We'll need to know how many sublevel is present. Therefore, the ground state electron configuration of the element, chlorine is: It has 17 electrons, filling up to the 3p orbital. Electron configuration chart of all elements is mentioned in the table below.. Chlorine Cl Electron Configuration.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Cl Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Therefore, the ground state electron configuration of the element, chlorine is: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Electron configuration chart of all elements is mentioned in the. Chlorine Cl Electron Configuration.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Cl Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. We'll need to know how many sublevel is present. Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵.. Chlorine Cl Electron Configuration.
From animalia-life.club
Chlorine Electron Configuration Chlorine Cl Electron Configuration Electron configuration of chlorine is [ne] 3s2 3p5. Therefore, the ground state electron configuration of the element, chlorine is: It is an extremely reactive element and a strong oxidising agent: The shorthand electron configuration (or noble gas configuration) as well as. We'll need to know how many sublevel is present. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. It has 17. Chlorine Cl Electron Configuration.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It is an extremely reactive element and a strong oxidising agent: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. The shorthand electron configuration (or noble gas configuration) as well as.. Chlorine Cl Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Cl Electron Configuration We'll need to know how many sublevel is present. The shorthand electron configuration (or noble gas configuration) as well as. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Therefore, the ground state electron configuration of the element, chlorine is: The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. It has 17 electrons, filling up to the 3p orbital. How to write the electron. Chlorine Cl Electron Configuration.
From www.pinterest.ca
How to Write the Orbital Diagram for Chlorine (Cl)? Electron Chlorine Cl Electron Configuration We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Therefore, the ground state electron configuration of the element, chlorine is: Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas. Chlorine Cl Electron Configuration.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Chlorine Cl Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It has 17 electrons, filling up to the 3p orbital. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we. Chlorine Cl Electron Configuration.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Cl Electron Configuration #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The shorthand electron configuration. Chlorine Cl Electron Configuration.
From drivenheisenberg.blogspot.com
Electron Dot Diagram For Chlorine Drivenheisenberg Chlorine Cl Electron Configuration The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. We'll need to know how many sublevel is present. It is an extremely reactive element and a strong oxidising agent: Electron configuration chart of. Chlorine Cl Electron Configuration.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Cl Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It is an extremely reactive element. Chlorine Cl Electron Configuration.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C026/3006 Science Photo Chlorine Cl Electron Configuration Electron configuration chart of all elements is mentioned in the table below. It has 17 electrons, filling up to the 3p orbital. The shorthand electron configuration (or noble gas configuration) as well as. It is an extremely reactive element and a strong oxidising agent: Therefore, the ground state electron configuration of the element, chlorine is: Electron configuration of chlorine is. Chlorine Cl Electron Configuration.
From api-project-1022638073839.appspot.com
What is a set of four quantum numbers that could represent the last Chlorine Cl Electron Configuration #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. It has 17 electrons, filling up to the 3p orbital. Electron configuration chart of all elements is mentioned in the table below. Chlorine has an atomic number of 17,. Chlorine Cl Electron Configuration.
From www.transtutors.com
(Get Answer) What is the electron configuration for Cl, chlorine, and Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The shorthand electron configuration (or. Chlorine Cl Electron Configuration.
From byjus.com
Electronic Configuration Distribution of Electrons Chemistry Chlorine Cl Electron Configuration Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. It has 17 electrons, filling up to the 3p orbital. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know. Chlorine Cl Electron Configuration.
From www.researchgate.net
Chlorine electron binding energies Download Table Chlorine Cl Electron Configuration We'll need to know how many sublevel is present. Therefore, the ground state electron configuration of the element, chlorine is: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. The shorthand electron configuration (or noble gas configuration) as well as. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. The electron configuration. Chlorine Cl Electron Configuration.
From ar.inspiredpencil.com
Orbital Diagram For Chlorine Chlorine Cl Electron Configuration #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Electron configuration of chlorine is [ne] 3s2 3p5. We'll need to know how many sublevel is present. The shorthand electron configuration (or noble gas configuration) as well as. Therefore, the ground state. Chlorine Cl Electron Configuration.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Cl Electron Configuration The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron. Chlorine Cl Electron Configuration.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Cl Electron Configuration Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It is an extremely reactive element and a strong oxidising agent: How to write the electron configuration for. Chlorine Cl Electron Configuration.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Cl Electron Configuration We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. Therefore, the ground state electron configuration of the element, chlorine is: The shorthand electron configuration (or noble gas configuration). Chlorine Cl Electron Configuration.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Cl Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. We'll need to know how many sublevel is present. It is an extremely reactive element and a strong oxidising agent: Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Electron configuration of chlorine is [ne] 3s2 3p5.. Chlorine Cl Electron Configuration.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. We'll need to know how many sublevel is present. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. The shorthand electron configuration (or noble gas configuration) as well. Chlorine Cl Electron Configuration.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Cl Electron Configuration Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Electron configuration chart of all elements is mentioned in the table below. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need. Chlorine Cl Electron Configuration.
From www.schoolmykids.com
Cl Chlorine Element Information Facts, Properties, Trends, Uses and Chlorine Cl Electron Configuration It is an extremely reactive element and a strong oxidising agent: The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. We'll need to know how many sublevel is present. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Chlorine has an. Chlorine Cl Electron Configuration.
From www.chegg.com
Solved Give the full electron configuration for chlorine Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. We'll need to know how many sublevel is present. It is an extremely reactive element and a strong oxidising agent: The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the. Chlorine Cl Electron Configuration.
From favpng.com
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. It is an extremely reactive element and a strong oxidising agent: Therefore, the ground state electron configuration of the element, chlorine is: #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. Electron configuration of chlorine is [ne] 3s2 3p5. The shorthand electron configuration (or noble gas configuration) as well as. How to. Chlorine Cl Electron Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Cl Electron Configuration Therefore, the ground state electron configuration of the element, chlorine is: How to write the electron configuration for chlorine (cl) in order to write the chlorine electron configuration we first need to know the number. #1s^2# #2s^2# #2p^6# #3s^2# #3p^5# = #color. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Chlorine has an atomic number of 17, which means it has. Chlorine Cl Electron Configuration.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Cl Electron Configuration It has 17 electrons, filling up to the 3p orbital. The electron configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Therefore, the ground state electron configuration of the element, chlorine is: Electron configuration of chlorine is [ne] 3s2 3p5. Electron configuration chart. Chlorine Cl Electron Configuration.