How Does Boron Form An Ion . This is balanced by 5 electrons. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Boron does not generally make ionic bonds, it forms stable covalent bonds. Two of them are core electrons and the remaining 3 are valence electrons. When mixed with water, the weakly acidic and electron deficient boric acid accepts. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). I.e., it conducts electricity like a metal at high temperatures and is. Boron has a charge of 5. 93 rows ionic charge: Pure crystalline boron is a black, lustrous semiconductor; Although opaque to visible light, boron can transmit portions of infrared light. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice.
from www.alamy.com
Although opaque to visible light, boron can transmit portions of infrared light. 93 rows ionic charge: Boron does not generally make ionic bonds, it forms stable covalent bonds. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Pure crystalline boron is a black, lustrous semiconductor; I.e., it conducts electricity like a metal at high temperatures and is. When mixed with water, the weakly acidic and electron deficient boric acid accepts. This electric charge generated on the ion is. This is balanced by 5 electrons. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice.
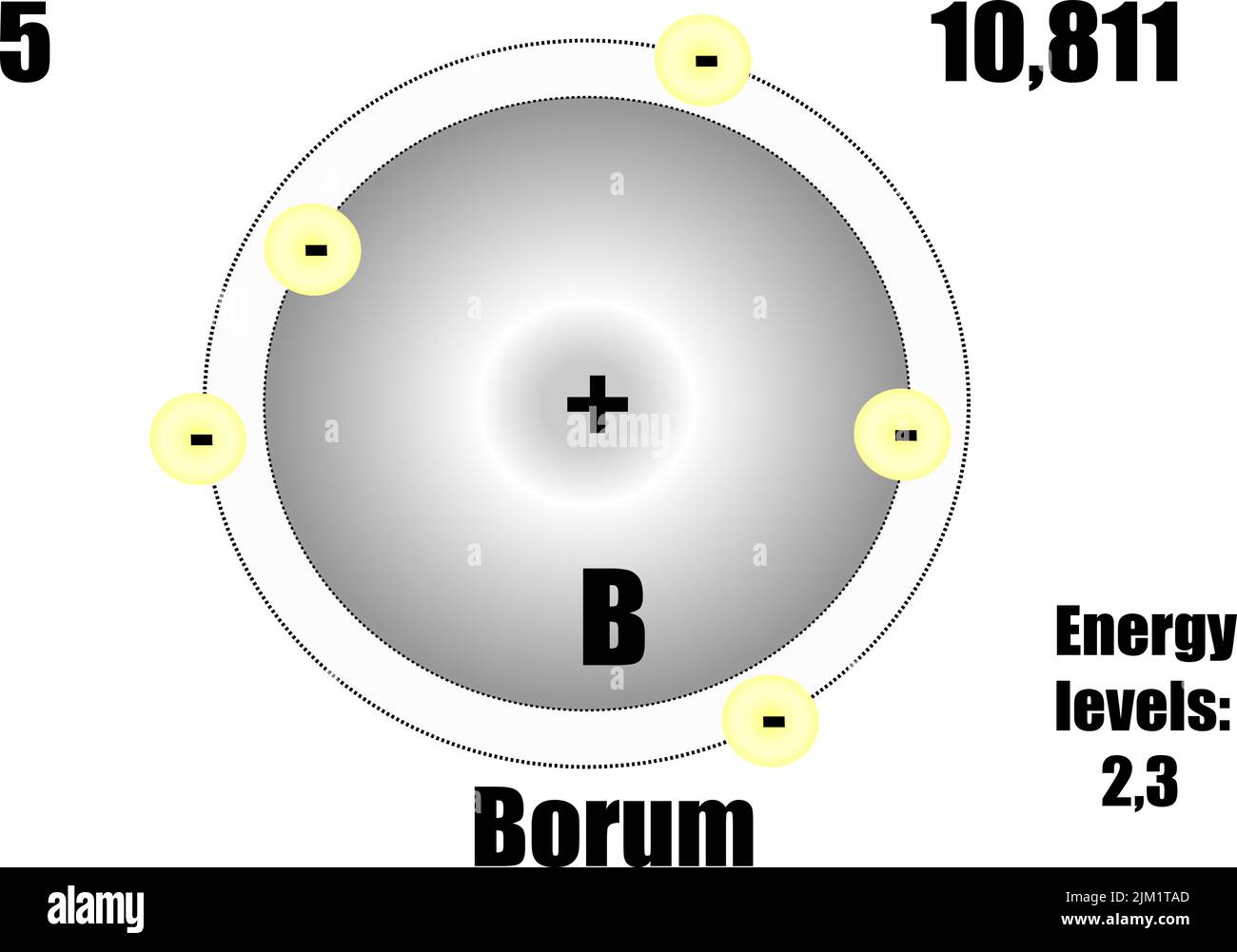
Boron atom, with mass and energy levels. Vector illustration Stock
How Does Boron Form An Ion Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. When mixed with water, the weakly acidic and electron deficient boric acid accepts. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Pure crystalline boron is a black, lustrous semiconductor; Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. This is balanced by 5 electrons. Boron has a charge of 5. I.e., it conducts electricity like a metal at high temperatures and is. This electric charge generated on the ion is. Two of them are core electrons and the remaining 3 are valence electrons. Although opaque to visible light, boron can transmit portions of infrared light. 93 rows ionic charge: Boron does not generally make ionic bonds, it forms stable covalent bonds.
From userdbcassandra.z19.web.core.windows.net
Lewis Dot Diagram For Boron How Does Boron Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When mixed with water, the weakly acidic and electron deficient boric acid accepts. 93 rows ionic charge: Although opaque to visible light, boron can transmit portions of infrared light. With its high ionization energy, low electron affinity, low electronegativity, and. How Does Boron Form An Ion.
From sciencenotes.org
Boron Atom Science Notes and Projects How Does Boron Form An Ion I.e., it conducts electricity like a metal at high temperatures and is. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. 93 rows ionic charge: Although opaque to visible light, boron can transmit portions of infrared light. Boron has a charge of 5. Two of them are core. How Does Boron Form An Ion.
From www.dreamstime.com
Diagram Representation of the Element Boron Stock Vector Illustration How Does Boron Form An Ion 93 rows ionic charge: I.e., it conducts electricity like a metal at high temperatures and is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Pure crystalline boron. How Does Boron Form An Ion.
From www.youtube.com
Atomic Structure (Bohr Model) for Boron (B) YouTube How Does Boron Form An Ion When mixed with water, the weakly acidic and electron deficient boric acid accepts. This electric charge generated on the ion is. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. I.e., it conducts electricity like a metal at high temperatures and is. Pure crystalline boron is a black,. How Does Boron Form An Ion.
From www.animalia-life.club
Boron Protons Neutrons Electrons How Does Boron Form An Ion Boron has a charge of 5. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. When mixed with water, the weakly acidic and electron deficient boric acid accepts. This is balanced by 5 electrons. Although opaque to visible light, boron can transmit portions of infrared light. Pure crystalline boron. How Does Boron Form An Ion.
From www.animalia-life.club
Boron Protons Neutrons Electrons How Does Boron Form An Ion When mixed with water, the weakly acidic and electron deficient boric acid accepts. Although opaque to visible light, boron can transmit portions of infrared light. This is balanced by 5 electrons. Two of them are core electrons and the remaining 3 are valence electrons. I.e., it conducts electricity like a metal at high temperatures and is. 93 rows ionic charge:. How Does Boron Form An Ion.
From www.showme.com
Boron Bohr model Science ShowMe How Does Boron Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Two of them are core electrons and the remaining 3 are valence electrons. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Boron occurs. How Does Boron Form An Ion.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline How Does Boron Form An Ion Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Pure crystalline boron is a black, lustrous semiconductor; This is balanced by 5 electrons. Two of them are core electrons and the remaining 3 are valence electrons. I.e., it conducts electricity like a metal at high temperatures and is. Boron. How Does Boron Form An Ion.
From www.youtube.com
Boron Electron Configuration YouTube How Does Boron Form An Ion This electric charge generated on the ion is. 93 rows ionic charge: Boron has a charge of 5. Pure crystalline boron is a black, lustrous semiconductor; When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Boron does not generally make ionic bonds, it forms stable covalent bonds. Although opaque. How Does Boron Form An Ion.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation ID6415669 How Does Boron Form An Ion With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Pure crystalline boron is a black, lustrous semiconductor; Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and. How Does Boron Form An Ion.
From downefile551.weebly.com
Boron Atom downefile How Does Boron Form An Ion When mixed with water, the weakly acidic and electron deficient boric acid accepts. Although opaque to visible light, boron can transmit portions of infrared light. Two of them are core electrons and the remaining 3 are valence electrons. Pure crystalline boron is a black, lustrous semiconductor; This electric charge generated on the ion is. This is balanced by 5 electrons.. How Does Boron Form An Ion.
From periodictableguide.com
Boron (B) Periodic Table (Element Information & More) How Does Boron Form An Ion Although opaque to visible light, boron can transmit portions of infrared light. When mixed with water, the weakly acidic and electron deficient boric acid accepts. Boron does not generally make ionic bonds, it forms stable covalent bonds. Two of them are core electrons and the remaining 3 are valence electrons. I.e., it conducts electricity like a metal at high temperatures. How Does Boron Form An Ion.
From www.animalia-life.club
Boron Protons Neutrons Electrons How Does Boron Form An Ion Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. When mixed with water, the weakly acidic and electron deficient boric acid accepts. Boron has a charge of 5. This is balanced by 5 electrons. When the atom. How Does Boron Form An Ion.
From asgburange.blogspot.com
BORON FOR UNDERGRADUATES How Does Boron Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. Two of them are core electrons and the remaining 3 are valence electrons. Pure crystalline boron is a black, lustrous semiconductor; When mixed with water, the weakly acidic and electron deficient boric. How Does Boron Form An Ion.
From visualizingchemstout.blogspot.com
Visualizing Chemistry 105 Activity 5 How Does Boron Form An Ion This electric charge generated on the ion is. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. When mixed with water, the weakly acidic and electron deficient boric acid accepts. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. How Does Boron Form An Ion.
From www.benjamin-mills.com
Electron arrangements How Does Boron Form An Ion Pure crystalline boron is a black, lustrous semiconductor; Although opaque to visible light, boron can transmit portions of infrared light. I.e., it conducts electricity like a metal at high temperatures and is. Boron has a charge of 5. 93 rows ionic charge: With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form. How Does Boron Form An Ion.
From www.youtube.com
How to find Protons and Electrons for B 3+ (Boron Ion) YouTube How Does Boron Form An Ion I.e., it conducts electricity like a metal at high temperatures and is. Boron has a charge of 5. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. When mixed with water, the weakly acidic and electron deficient boric acid accepts. 93. How Does Boron Form An Ion.
From www.sciencephoto.com
Boron, atomic structure Stock Image C013/1499 Science Photo Library How Does Boron Form An Ion Boron has a charge of 5. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. 93 rows ionic charge: Pure crystalline boron is a black, lustrous semiconductor; Although opaque to visible light, boron can transmit portions of infrared light. With its high ionization energy, low electron affinity, low electronegativity,. How Does Boron Form An Ion.
From borates.today
Alkaline Boron A Balancing Force In Nature How Does Boron Form An Ion Two of them are core electrons and the remaining 3 are valence electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Boron has a charge of 5. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not. How Does Boron Form An Ion.
From www.animalia-life.club
Boron Protons Neutrons Electrons How Does Boron Form An Ion Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Although opaque to visible light, boron can transmit portions of infrared light. Two of them are core electrons and the remaining 3 are valence electrons. 93 rows ionic charge: This is balanced by 5 electrons. I.e., it conducts electricity like. How Does Boron Form An Ion.
From borates.today
Boron Electron Valence Borates Today How Does Boron Form An Ion Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. 93 rows ionic charge: When mixed with water, the weakly acidic and electron deficient boric acid accepts. Although opaque to visible light, boron can transmit portions of infrared light. Boron has a charge of 5. Two of them are core. How Does Boron Form An Ion.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist How Does Boron Form An Ion Boron does not generally make ionic bonds, it forms stable covalent bonds. Pure crystalline boron is a black, lustrous semiconductor; With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Boron has a charge of 5. Boron occurs as an orthoboric acid in some volcanic spring waters, and as. How Does Boron Form An Ion.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free How Does Boron Form An Ion With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. 93 rows ionic charge: When mixed with water, the weakly acidic and electron deficient boric acid accepts. This electric charge generated on the ion is. This is balanced by 5 electrons. Boron has a charge of 5. Two of. How Does Boron Form An Ion.
From periodictable.me
How To Find The Boron Electron Configuration (B) How Does Boron Form An Ion Boron has a charge of 5. Two of them are core electrons and the remaining 3 are valence electrons. Boron does not generally make ionic bonds, it forms stable covalent bonds. 93 rows ionic charge: With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. When mixed with water,. How Does Boron Form An Ion.
From www.alamy.com
Diagram representation of the element boron illustration Stock Vector How Does Boron Form An Ion I.e., it conducts electricity like a metal at high temperatures and is. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Two of them are core electrons and the remaining 3 are valence electrons. When mixed with water, the weakly acidic and electron deficient boric acid accepts. 93 rows. How Does Boron Form An Ion.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) How Does Boron Form An Ion Boron does not generally make ionic bonds, it forms stable covalent bonds. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. This is balanced by 5 electrons. This electric charge generated on the ion is. I.e., it conducts electricity like a metal at high temperatures and is. Two of. How Does Boron Form An Ion.
From www.animalia-life.club
Boron Element How Does Boron Form An Ion Boron does not generally make ionic bonds, it forms stable covalent bonds. This electric charge generated on the ion is. This is balanced by 5 electrons. 93 rows ionic charge: Two of them are core electrons and the remaining 3 are valence electrons. When mixed with water, the weakly acidic and electron deficient boric acid accepts. Boron occurs as an. How Does Boron Form An Ion.
From www.britannica.com
boron Properties, Uses, & Facts Britannica How Does Boron Form An Ion Pure crystalline boron is a black, lustrous semiconductor; When mixed with water, the weakly acidic and electron deficient boric acid accepts. This electric charge generated on the ion is. Boron has a charge of 5. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. This is balanced by. How Does Boron Form An Ion.
From www.alamy.com
Boron atom, with mass and energy levels. Vector illustration Stock How Does Boron Form An Ion With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. This electric charge generated on the ion is. I.e., it conducts electricity like a metal at high temperatures and is. Although opaque to visible light, boron can transmit portions of infrared light. When the atom loses or gains one. How Does Boron Form An Ion.
From mungfali.com
Boron Electron Dot Diagram How Does Boron Form An Ion When mixed with water, the weakly acidic and electron deficient boric acid accepts. Although opaque to visible light, boron can transmit portions of infrared light. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. 93 rows ionic charge: I.e., it conducts electricity like a metal at high temperatures and. How Does Boron Form An Ion.
From www.alamy.com
3d render of atom structure of boron isolated over white background How Does Boron Form An Ion Although opaque to visible light, boron can transmit portions of infrared light. 93 rows ionic charge: This electric charge generated on the ion is. Boron does not generally make ionic bonds, it forms stable covalent bonds. Pure crystalline boron is a black, lustrous semiconductor; With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. How Does Boron Form An Ion.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table How Does Boron Form An Ion Boron has a charge of 5. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. Boron does not generally make ionic bonds, it forms stable covalent bonds. This is balanced by 5 electrons. Although opaque to visible light, boron can transmit portions of infrared light. Pure crystalline boron is. How Does Boron Form An Ion.
From material-properties.org
Boron Periodic Table and Atomic Properties How Does Boron Form An Ion This electric charge generated on the ion is. Although opaque to visible light, boron can transmit portions of infrared light. Boron has a charge of 5. This is balanced by 5 electrons. Pure crystalline boron is a black, lustrous semiconductor; 93 rows ionic charge: With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does. How Does Boron Form An Ion.
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds How Does Boron Form An Ion 93 rows ionic charge: Pure crystalline boron is a black, lustrous semiconductor; When mixed with water, the weakly acidic and electron deficient boric acid accepts. Boron occurs as an orthoboric acid in some volcanic spring waters, and as borates in the minerals borax and colemanite. I.e., it conducts electricity like a metal at high temperatures and is. This is balanced. How Does Boron Form An Ion.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library How Does Boron Form An Ion This electric charge generated on the ion is. Boron has a charge of 5. Although opaque to visible light, boron can transmit portions of infrared light. When mixed with water, the weakly acidic and electron deficient boric acid accepts. Two of them are core electrons and the remaining 3 are valence electrons. Boron occurs as an orthoboric acid in some. How Does Boron Form An Ion.