How Does Bromine Form An Ion . Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. For elements in groups 6 and 7, the charge on the. Shiny element that is a good conductor of electricity and heat, and which. Positive and negative ions form when a. This electric charge generated on the ion is. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge:
from www.americanelements.com
This electric charge generated on the ion is. Positive and negative ions form when a. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). For elements in groups 6 and 7, the charge on the. 93 rows ionic charge: The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Shiny element that is a good conductor of electricity and heat, and which.
Bromine (Br) AMERICAN ELEMENTS
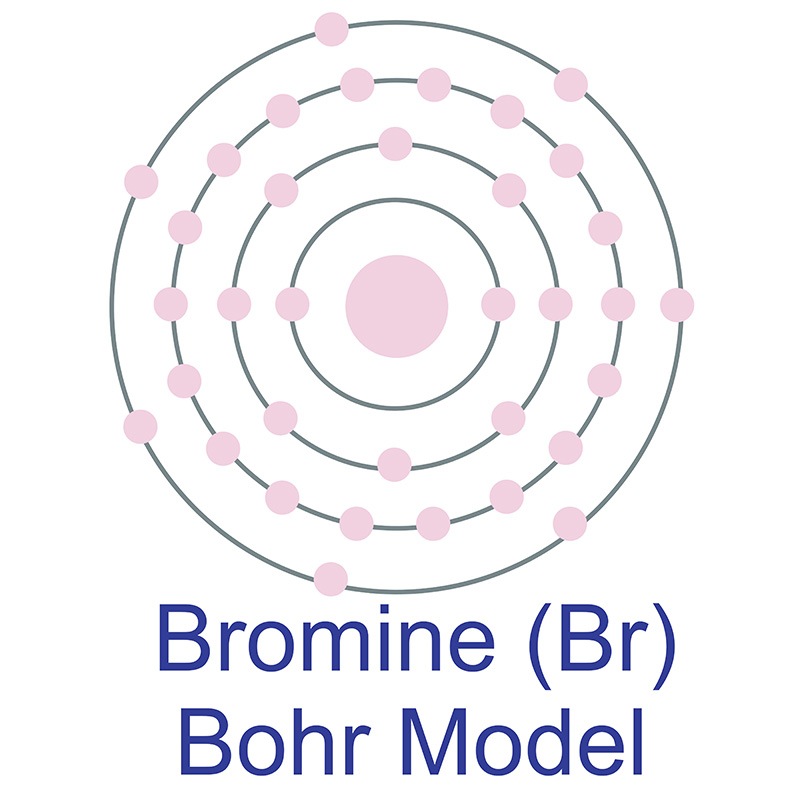
How Does Bromine Form An Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. This electric charge generated on the ion is. Shiny element that is a good conductor of electricity and heat, and which. For elements in groups 6 and 7, the charge on the. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. 93 rows ionic charge:
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of How Does Bromine Form An Ion Positive and negative ions form when a. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For elements in groups 6. How Does Bromine Form An Ion.
From www.animalia-life.club
Electron Configuration For Bromine How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Positive and negative ions form when a. For elements in groups 6 and 7, the charge on the. 93 rows ionic charge: The ions. How Does Bromine Form An Ion.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic How Does Bromine Form An Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Positive and negative ions form when a. 93 rows ionic charge: This electric charge generated on the ion is. Shiny element that is a good conductor of electricity and heat, and which. Note, members of. How Does Bromine Form An Ion.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Shiny element that is a good conductor of electricity and heat, and which. Positive and negative ions form when a. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is. How Does Bromine Form An Ion.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: For elements in groups 6 and 7, the charge on the. When the. How Does Bromine Form An Ion.
From www.animalia-life.club
Electron Configuration For Bromine How Does Bromine Form An Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. This electric charge generated on the ion is. Positive and negative ions form when a. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. How Does Bromine Form An Ion.
From mungfali.com
Aufbau Diagram For Bromine How Does Bromine Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: Positive and negative. How Does Bromine Form An Ion.
From app.emaze.com
Bromine ) on emaze How Does Bromine Form An Ion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ions have the electronic structure of a noble gas (group 0 element), with a full outer. How Does Bromine Form An Ion.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. 93 rows ionic charge: Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. For elements in groups 6 and 7, the charge on the. The first ionization energy of bromine is high, and. How Does Bromine Form An Ion.
From www.gauthmath.com
Solved How many protons, electron and neutron does bromine ion have How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. This electric charge generated on the ion is. Positive and negative ions form when a. When the atom loses. How Does Bromine Form An Ion.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Shiny element that is a good conductor of electricity and heat, and which. 93 rows ionic charge: The first ionization energy of bromine is. How Does Bromine Form An Ion.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library How Does Bromine Form An Ion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. This electric charge generated on the ion is. Positive and negative ions form when a. For elements in groups 6 and 7, the charge on the. Shiny element that is a good conductor of electricity and heat,. How Does Bromine Form An Ion.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. 93. How Does Bromine Form An Ion.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. This electric charge generated on the ion is. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Shiny element that is a good conductor of electricity. How Does Bromine Form An Ion.
From www.americanelements.com
Bromine (Br) AMERICAN ELEMENTS How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. Positive and negative ions form when a. Note, members of the same family tend to form similar compounds, so. How Does Bromine Form An Ion.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine How Does Bromine Form An Ion 93 rows ionic charge: This electric charge generated on the ion is. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. When the atom loses or gains one. How Does Bromine Form An Ion.
From dreamstime.com
Bromine Form Periodic Table Of Elements Stock Illustration Image 7137169 How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. For elements in groups 6 and 7, the charge on the. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Positive and negative ions form when a. The. How Does Bromine Form An Ion.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. 93 rows ionic charge:. How Does Bromine Form An Ion.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained How Does Bromine Form An Ion This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. For elements in groups 6 and 7,. How Does Bromine Form An Ion.
From www.animalia-life.club
Electron Configuration For Bromine How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is. How Does Bromine Form An Ion.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. How Does Bromine Form An Ion.
From www.chegg.com
Solved The correct mechanism for the addition of bromine to How Does Bromine Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine. How Does Bromine Form An Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram How Does Bromine Form An Ion The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. This electric charge generated on the ion is. Shiny element that is a good conductor of electricity and heat, and which. For elements in groups 6 and 7, the charge on the. When the atom loses or gains one or more electrons,. How Does Bromine Form An Ion.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron How Does Bromine Form An Ion Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: Shiny element that is a good. How Does Bromine Form An Ion.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica How Does Bromine Form An Ion Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Shiny element that is a good conductor of electricity and heat, and which. The first ionization energy. How Does Bromine Form An Ion.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). For elements in groups 6 and 7, the charge on the. This electric charge generated on the ion is. The first ionization energy of bromine is high,. How Does Bromine Form An Ion.
From ar.inspiredpencil.com
Atomic Structure Of Bromine How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. This electric charge generated on the ion is. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen. How Does Bromine Form An Ion.
From www.alamy.com
Bromine molecule, illustration Stock Photo Alamy How Does Bromine Form An Ion 93 rows ionic charge: Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Shiny element that is a good conductor of electricity and heat, and which. Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element),. How Does Bromine Form An Ion.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) How Does Bromine Form An Ion Shiny element that is a good conductor of electricity and heat, and which. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. This electric charge generated on the ion is. The. How Does Bromine Form An Ion.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. 93 rows ionic charge: Positive and. How Does Bromine Form An Ion.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Positive and negative ions form when a. This. How Does Bromine Form An Ion.
From www.vectorstock.com
Bromine chemical element Royalty Free Vector Image How Does Bromine Form An Ion Positive and negative ions form when a. The ions have the electronic structure of a noble gas (group 0 element), with a full outer shell. For elements in groups 6 and 7, the charge on the. This electric charge generated on the ion is. Note, members of the same family tend to form similar compounds, so bromine and iodine form. How Does Bromine Form An Ion.
From material-properties.org
Bromine Periodic Table and Atomic Properties How Does Bromine Form An Ion For elements in groups 6 and 7, the charge on the. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Positive and negative ions form when a. Shiny element that is a good conductor of electricity and heat, and which. The first ionization energy of bromine is high, and. How Does Bromine Form An Ion.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare How Does Bromine Form An Ion The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. 93 rows ionic charge: The ions have the electronic structure of a. How Does Bromine Form An Ion.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds How Does Bromine Form An Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. 93 rows ionic charge: Positive and negative ions form when a. The ions have the. How Does Bromine Form An Ion.