Does Snow Salt Melt Ice . Salt doesn’t directly melt ice, nor does it make snow simply disappear. First, it’s important to understand a bit about h 2 o in the winter. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. But how does salt do it? If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. This is because salt is used to melt the ice and snow and keep it from. A chemist explains how salt affects water and ice.
from snowicesalt.com
More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. But how does salt do it? Salt doesn’t directly melt ice, nor does it make snow simply disappear. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. A chemist explains how salt affects water and ice. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. This is because salt is used to melt the ice and snow and keep it from. First, it’s important to understand a bit about h 2 o in the winter. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water.
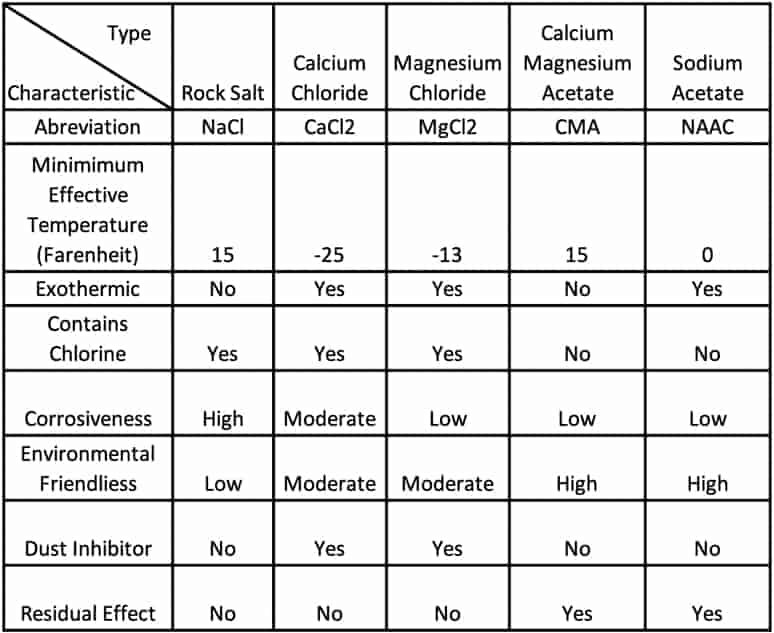
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC, A RASEVIC COMPANY
Does Snow Salt Melt Ice First, it’s important to understand a bit about h 2 o in the winter. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. But how does salt do it? First, it’s important to understand a bit about h 2 o in the winter. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. A chemist explains how salt affects water and ice. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. This is because salt is used to melt the ice and snow and keep it from. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Salt doesn’t directly melt ice, nor does it make snow simply disappear.
From www.dreamstime.com
Ice Melt Salt Melting Ice and Snow on Sidewalk Stock Photo Image of home, freezing 240902002 Does Snow Salt Melt Ice But how does salt do it? First, it’s important to understand a bit about h 2 o in the winter. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. The actual reason that the application. Does Snow Salt Melt Ice.
From safethaw.com
How Exactly Does Salt Melt Ice? An Exploration Does Snow Salt Melt Ice Salt doesn’t directly melt ice, nor does it make snow simply disappear. But how does salt do it? if you live in a city that gets lots of snow and ice, then you're familiar with road salt. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a. Does Snow Salt Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice Faster Than Sugar? SCIENCE EXPLAINED Does Snow Salt Melt Ice A chemist explains how salt affects water and ice. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. This is because salt is used to melt the ice and snow and keep it from. if you live in a city that gets lots of snow and ice, then you're. Does Snow Salt Melt Ice.
From chemistry.about.com
Why Does Salt Melt Ice? Understanding How It Works Does Snow Salt Melt Ice A chemist explains how salt affects water and ice. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. Instead. Does Snow Salt Melt Ice.
From www.ebay.com
Splash Ice Melt Safe Snow & Ice Melter Salt Pellets For Sidewalks Steps 12 Lb eBay Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. But how does salt do it? Instead it makes water less likely to freeze in a phenomenon called freezing point depression. A chemist explains how salt affects water and ice. The actual reason that the application of salt causes ice to melt is that. Does Snow Salt Melt Ice.
From www.alamy.com
Ice melt salt melting ice and snow on sidewalk. Snow removal, melting ice and winter weather Does Snow Salt Melt Ice First, it’s important to understand a bit about h 2 o in the winter. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. But how does salt do it? The actual reason that the application of salt causes ice to melt is that a solution of water and. Does Snow Salt Melt Ice.
From fox17.com
Behind the Science Why Salt is Used to Melt Ice WZTV Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. If you live in an area with a cold and icy winter, you have probably experienced. Does Snow Salt Melt Ice.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC, A RASEVIC COMPANY Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Salt doesn’t directly melt ice, nor does it make snow simply. Does Snow Salt Melt Ice.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Does Snow Salt Melt Ice But how does salt do it? if you live in a city that gets lots of snow and ice, then you're familiar with road salt. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. More than 20 million tons of salt are used every year to melt snow. Does Snow Salt Melt Ice.
From www.farmersalmanac.com
How to Melt Ice Naturally Farmers' Almanac Plan Your Day. Grow Your Life. Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. First, it’s important to understand a bit about h 2 o. Does Snow Salt Melt Ice.
From laptrinhx.com
How does salt melt snow? LaptrinhX / News Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. First, it’s important to understand a bit about h 2 o in the winter. The actual reason that the application of salt causes ice to melt is that a. Does Snow Salt Melt Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. First, it’s important to understand a bit about h 2 o in the winter. More than 20 million tons of salt are used every year to melt snow and ice in. Does Snow Salt Melt Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Does Snow Salt Melt Ice If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. But how does salt do it? Salt doesn’t directly melt ice, nor does it make snow simply disappear. A chemist explains how salt affects water and ice. The actual reason that the application of salt causes ice to melt. Does Snow Salt Melt Ice.
From www.pinterest.com
SNOW JOE MELT Sodium Rock Salt Ice Melter Works to 15F Bagged 50 LBS 20800418 HSN in 2022 Does Snow Salt Melt Ice More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. A chemist explains how salt affects water and ice. The actual reason that the application of salt causes ice to melt. Does Snow Salt Melt Ice.
From ninjadeicer.com
Rock Salt vs Ice Melt What’s the Difference? Ninja DeIcer Does Snow Salt Melt Ice First, it’s important to understand a bit about h 2 o in the winter. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. If you live in an area with a cold and icy winter,. Does Snow Salt Melt Ice.
From www.copecompany.com
Why & How Does Salt Melt Ice? Cope Company Salt Does Snow Salt Melt Ice if you live in a city that gets lots of snow and ice, then you're familiar with road salt. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. First, it’s important to understand a bit about h 2 o in. Does Snow Salt Melt Ice.
From www.pinterest.nz
Here's A ScienceBacked Guide To DeIcing Your Driveway Salt and ice, Melting salt, Snow melting Does Snow Salt Melt Ice if you live in a city that gets lots of snow and ice, then you're familiar with road salt. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. More than 20 million tons of salt are used every year to. Does Snow Salt Melt Ice.
From edenapp.com
7 Ways to Melt Ice without Salt or Ice Melt Eden Lawn Care and Snow Removal Does Snow Salt Melt Ice If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. First, it’s important to. Does Snow Salt Melt Ice.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. A chemist explains how salt affects water and ice. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Salt doesn’t directly melt ice, nor does it make snow simply disappear. The actual reason. Does Snow Salt Melt Ice.
From northrockmineral.com
Ice Melt vs. Rock Salt Snow Joe, LLC. Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. But how does salt do it? More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. First, it’s important to understand. Does Snow Salt Melt Ice.
From www.thoughtco.com
How Salt Melts Ice and Snow Does Snow Salt Melt Ice Instead it makes water less likely to freeze in a phenomenon called freezing point depression. But how does salt do it? If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. Salt doesn’t directly melt ice, nor does it make snow simply disappear. if you live in a city. Does Snow Salt Melt Ice.
From www.bustle.com
Does Salt Melt Snow? Here's What You Need To Know If You're Trying To Clear Your Own Path Does Snow Salt Melt Ice First, it’s important to understand a bit about h 2 o in the winter. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. A chemist explains how salt affects water. Does Snow Salt Melt Ice.
From www.britannica.com
Why Does Salt Melt Ice? Britannica Does Snow Salt Melt Ice Instead it makes water less likely to freeze in a phenomenon called freezing point depression. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. But how does salt do it? A chemist explains how salt affects water and ice. Salt doesn’t directly melt ice, nor does it make. Does Snow Salt Melt Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Does Snow Salt Melt Ice The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. First, it’s important to understand a bit about h 2 o in the winter. if you live in a city that gets lots of snow and ice, then you're familiar with. Does Snow Salt Melt Ice.
From snappys-outdoor.com
What type of salt should you use this winter? Snappy's Outdoor Equipment Does Snow Salt Melt Ice A chemist explains how salt affects water and ice. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. This is because salt is used to melt the ice and snow and keep it from. More than 20 million tons of salt are used every year to melt snow. Does Snow Salt Melt Ice.
From www.alamy.com
A woman throwing salt on a driveway to melt the ice and snow Stock Photo Alamy Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Salt doesn’t directly melt ice, nor does it make snow simply disappear. If you live. Does Snow Salt Melt Ice.
From www.homedepot.com
Snow Joe Melt 50 lbs. Sodium Rock Salt Ice Melt MELT50RS The Home Depot Does Snow Salt Melt Ice First, it’s important to understand a bit about h 2 o in the winter. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. A chemist explains how salt affects water and ice. Salt. Does Snow Salt Melt Ice.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Scientific American Does Snow Salt Melt Ice A chemist explains how salt affects water and ice. But how does salt do it? if you live in a city that gets lots of snow and ice, then you're familiar with road salt. First, it’s important to understand a bit about h 2 o in the winter. If you live in an area with a cold and icy winter,. Does Snow Salt Melt Ice.
From www.youtube.com
How Does Salt Melt Ice? YouTube Does Snow Salt Melt Ice More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. Instead it makes water less likely to freeze in a phenomenon. Does Snow Salt Melt Ice.
From www.cleanlink.com
Ice Melt Do's and Don'ts Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. But how does salt do it? First, it’s important to understand a bit about h 2 o in the winter. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. More than 20. Does Snow Salt Melt Ice.
From www.uline.ca
Ice Melt, Rock Salt, Ice Melter, Snow Salt in Stock ULINE.ca Does Snow Salt Melt Ice Instead it makes water less likely to freeze in a phenomenon called freezing point depression. Salt doesn’t directly melt ice, nor does it make snow simply disappear. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. If you live in an area with a cold and icy winter, you have. Does Snow Salt Melt Ice.
From huntingwaterfalls.com
Why Does Salt Melt Ice? (Simple Science Explained) Does Snow Salt Melt Ice If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. First, it’s important to understand a bit about h 2 o in the winter. Instead it makes water less likely to freeze in a phenomenon called freezing point depression. Salt doesn’t directly melt ice, nor does it make snow. Does Snow Salt Melt Ice.
From www.dreamstime.com
Ice Melt Rock Salt is Being Spread on Your Walking Path To Melt the Snow and Ice Stock Photo Does Snow Salt Melt Ice If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. But how does salt do it? Salt doesn’t directly melt ice, nor does it make snow simply disappear. The actual. Does Snow Salt Melt Ice.
From www.homedepot.com
Snow Joe Melt 50 lbs. Sodium Rock Salt Ice Melt MELT50RS The Home Depot Does Snow Salt Melt Ice if you live in a city that gets lots of snow and ice, then you're familiar with road salt. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. A chemist explains how salt affects water and ice. More than 20 million tons of salt are used every year. Does Snow Salt Melt Ice.
From www.alamy.com
Ice melt salt melting ice and snow on sidewalk. Snow removal, melting ice and winter weather Does Snow Salt Melt Ice This is because salt is used to melt the ice and snow and keep it from. if you live in a city that gets lots of snow and ice, then you're familiar with road salt. If you live in an area with a cold and icy winter, you have probably experienced salt on sidewalks and roads. The actual reason that. Does Snow Salt Melt Ice.