Standard Molar Heat Of Formation Of Water . The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25.
from eduinput.com
Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. A pressure of 1 atm for gases and a concentration. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.
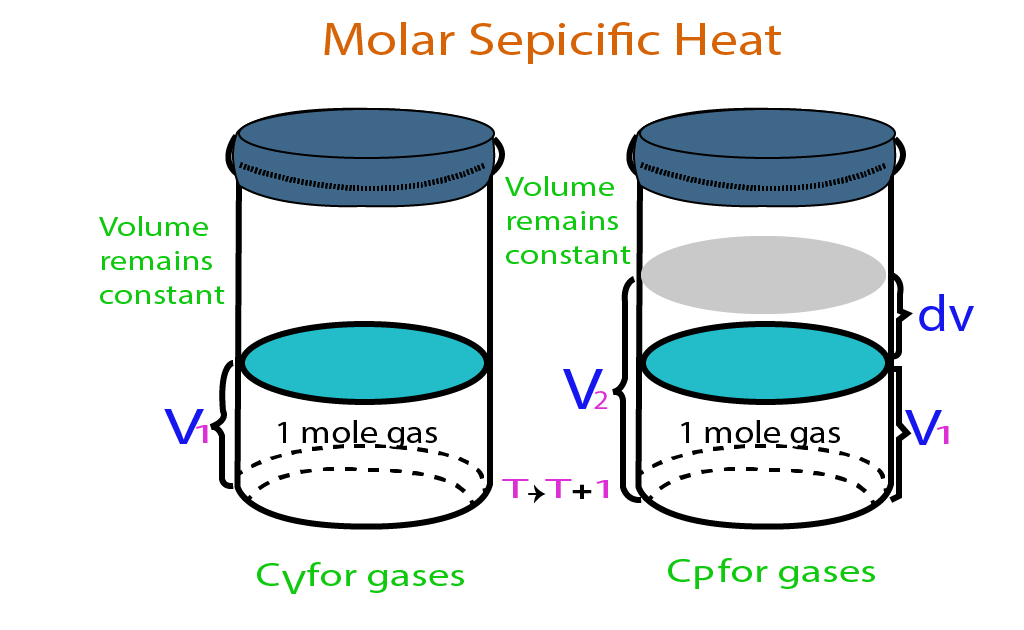
Molar Specific HeatSpecific heat, And Types
Standard Molar Heat Of Formation Of Water Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. A pressure of 1 atm for gases and a concentration. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.
From www.toppr.com
25. The standard molar heat of formation of ethane, carbon dioxide and water (liquid) are 21.1 Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: A pressure of 1 atm for gases and a concentration. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Molar Heat Of Formation Of Water.
From byjus.com
under the same conditions heat of formation of water and CO2 are 285, 394 kj /mol the heat of Standard Molar Heat Of Formation Of Water A pressure of 1 atm for gases and a concentration. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms. Standard Molar Heat Of Formation Of Water.
From www.youtube.com
Molar Enthalpy Calculations YouTube Standard Molar Heat Of Formation Of Water A pressure of 1 atm for gases and a concentration. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Molar Heat Of Formation Of Water.
From www.numerade.com
SOLVED What is the standard molar heat of solution for the aqueous dissolution of calcium Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Definition. Standard Molar Heat Of Formation Of Water.
From edurev.in
The standard molar heat of formation of ethane ,CO and water (l) are respectively 21.0,94.1 Standard Molar Heat Of Formation Of Water Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A pressure of 1 atm for gases and a concentration. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure. Standard Molar Heat Of Formation Of Water.
From www.toppr.com
The standard molar heat of formation of ethane, CO2 and water (l) are respectively 21.1 , 94 Standard Molar Heat Of Formation Of Water A pressure of 1 atm for gases and a concentration. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole. Standard Molar Heat Of Formation Of Water.
From mungfali.com
Standard Molar Enthalpy Of Formation Table Standard Molar Heat Of Formation Of Water Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: 193 rows in. Standard Molar Heat Of Formation Of Water.
From www.youtube.com
The standard molar heats of formation of ethane, carbon dioxide, and liquid water ate `21.1, 9 Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Also called standard. Standard Molar Heat Of Formation Of Water.
From narodnatribuna.info
Molar Enthalpy Calculations Youtube Standard Molar Heat Of Formation Of Water Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A pressure of 1 atm for gases and a concentration. The standard enthalpy of formation is a measure. Standard Molar Heat Of Formation Of Water.
From www.toppr.com
89. If standard molar enthalpy of formation of carbon dioxide gas, liquid water and ethane gas Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. A pressure of 1 atm for. Standard Molar Heat Of Formation Of Water.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Standard enthalpy change. Standard Molar Heat Of Formation Of Water.
From www.doubtnut.com
From the following equations, calculate the standard molar heat of formation of AgCl at 25^()C Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Molar Heat Of Formation Of Water.
From edurev.in
The standard molar heat of formation of ethane ,CO and water (l) are respectively 21.0,94.1 Standard Molar Heat Of Formation Of Water Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms. Standard Molar Heat Of Formation Of Water.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Standard Molar Heat Of Formation Of Water Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Definition and explanation of. Standard Molar Heat Of Formation Of Water.
From edurev.in
The standard molar heat of formation of ethane ,CO and water (l) are respectively 21.0,94.1 Standard Molar Heat Of Formation Of Water Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure. Standard Molar Heat Of Formation Of Water.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Molar Heat Of Formation Of Water Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Molar Heat Of Formation Of Water.
From www.chegg.com
Solved The standard molar heat of formation of water is Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is. Standard Molar Heat Of Formation Of Water.
From eduinput.com
Molar Specific HeatSpecific heat, And Types Standard Molar Heat Of Formation Of Water Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of. Standard Molar Heat Of Formation Of Water.
From www.toppr.com
The standard molar heat of formation of ethane, CO2 and water are respectively 21.1 , 94.1 Standard Molar Heat Of Formation Of Water Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data. Standard Molar Heat Of Formation Of Water.
From wizedu.com
The standard molar heat of formation of water is 258.8 kJ/mol.a. What is the significance of Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. 193 rows in chemistry and thermodynamics,. Standard Molar Heat Of Formation Of Water.
From www.toppr.com
The standard molar heats of formation of ethane, carbon dioxide and liquid water are 21.1 Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Definition and explanation of. Standard Molar Heat Of Formation Of Water.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Molar Heat Of Formation Of Water Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Molar Heat Of Formation Of Water.
From www.chegg.com
Solved The standard molar heat of formation of water is Standard Molar Heat Of Formation Of Water Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of. Standard Molar Heat Of Formation Of Water.
From www.chegg.com
Solved Table 6.2. Selected Standard Molar Enthalpies of Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: The standard enthalpy of. Standard Molar Heat Of Formation Of Water.
From www.chegg.com
Solved The standard enthalpy of formation of H2O(g) at 298 K Standard Molar Heat Of Formation Of Water Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a concentration. Also called standard enthalpy of formation, the molar heat of formation of a compound. Standard Molar Heat Of Formation Of Water.
From www.numerade.com
SOLVED Why is the standard molar enthalpy of formation, ΔHf^0 for liquid water different than Standard Molar Heat Of Formation Of Water A pressure of 1 atm for gases and a concentration. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Molar Heat Of Formation Of Water.
From www.youtube.com
The standard molar heat of formation of ethane CO_2 and water (l) are respectively 21.1 ,94.1 Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A pressure of 1 atm for gases and a concentration. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy change of formation (data. Standard Molar Heat Of Formation Of Water.
From www.doubtnut.com
The standard molar enthalpy of formation of ethane, carbon dioxide and Standard Molar Heat Of Formation Of Water Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: The standard enthalpy. Standard Molar Heat Of Formation Of Water.
From brainly.in
The standard molar heat of formation of ethane(g), CO2(g) and water(ℓ) are respectively 21.1 Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f). Standard Molar Heat Of Formation Of Water.
From byjus.com
The standard molar heat for formation ofethane, carbondioxide and water are respectively, 21.1 Standard Molar Heat Of Formation Of Water Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A pressure of 1 atm for gases and a concentration. Standard enthalpies of formation (\. Standard Molar Heat Of Formation Of Water.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3528115 Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh. Standard Molar Heat Of Formation Of Water.
From askfilo.com
11. The standard molar heat of formation of ethane, CO2 and water are re.. Standard Molar Heat Of Formation Of Water Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when. Standard Molar Heat Of Formation Of Water.
From www.toppr.com
The standard molar heat of formation of ethane , carbon dioxide and liquid water are 21.1 Standard Molar Heat Of Formation Of Water 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Also called standard enthalpy of formation, the molar heat of formation of a. Standard Molar Heat Of Formation Of Water.
From www.numerade.com
SOLVED Using the standard molar enthalpies of formation given, calculate the standard enthalpy Standard Molar Heat Of Formation Of Water The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Molar Heat Of Formation Of Water.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation, free download ID2964088 Standard Molar Heat Of Formation Of Water A pressure of 1 atm for gases and a concentration. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Standard enthalpies of formation (\ (δh^o_ {f}\)) are determined under standard conditions: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Molar Heat Of Formation Of Water.