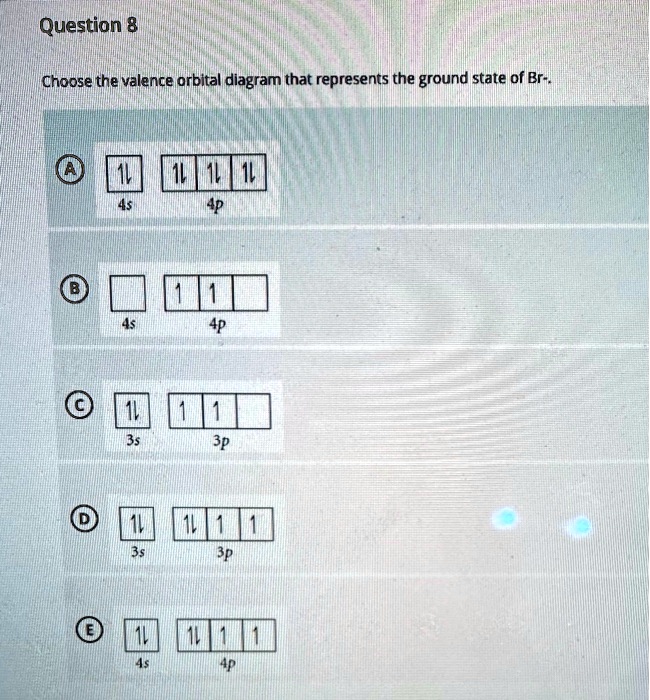
Bromine Ground State Configuration . The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The complete electron configuration of the bromine is: Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures.
from ar.inspiredpencil.com
Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The complete electron configuration of the bromine is: The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the.
Orbital Diagram Of Bromine
Bromine Ground State Configuration The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The complete electron configuration of the bromine is: Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. From the electrons in an atom, to the. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. From the electrons in an atom, to the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#.
From www.slideserve.com
PPT H o m e w o r k PowerPoint Presentation, free download ID3212314 Bromine Ground State Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: 1s 2 2s 2 2p. Bromine Ground State Configuration.
From www.numerade.com
SOLVED Write ground state electron configuration for following Bromine Ground State Configuration Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The complete electron configuration of the bromine is: From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The ground state electron configuration. Bromine Ground State Configuration.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Ground State Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. From the electrons in an atom, to the. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. The complete electron configuration of the. Bromine Ground State Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Ground State Configuration From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The complete electron configuration of the bromine is:. Bromine Ground State Configuration.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. The complete electron configuration of the bromine is: The electron configuration of bromine is. Bromine Ground State Configuration.
From www.numerade.com
SOLVED The complete groundstate electron configuration of aluminum Bromine Ground State Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10. Bromine Ground State Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Bromine Ground State Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The complete electron configuration of the bromine is: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The way we. Bromine Ground State Configuration.
From answers.yahoo.com
What is the groundstate electron configuration of bromine? Yahoo Answers Bromine Ground State Configuration From the electrons in an atom, to the. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The way we. Bromine Ground State Configuration.
From www.chegg.com
Solved Show the full groundstate electron configuration of Bromine Ground State Configuration From the electrons in an atom, to the. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#.. Bromine Ground State Configuration.
From www.numerade.com
SOLVEDDetermine the groundstate electron configuration of the Bromine Ground State Configuration This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. 1s 2 2s 2 2p 6 3s 2. Bromine Ground State Configuration.
From www.researchgate.net
Calculated groundstate bromine 4s, 4p σ and 4p π projected density of Bromine Ground State Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The complete electron configuration of the bromine is: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. From the electrons in an atom, to the. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar. Bromine Ground State Configuration.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Bromine Ground State Configuration From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The way we designate electronic configurations for cations. Bromine Ground State Configuration.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Ground State Configuration Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the. The complete electron configuration of the bromine is: Bromine atoms have 35 electrons and. Bromine Ground State Configuration.
From www.sketchite.com
Bromine Electron Valence Electrons Configuration Orbital Diagram Br Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The complete. Bromine Ground State Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Ground State Configuration The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons in an atom, to the. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Ground state electron configurations are the foundation for. Bromine Ground State Configuration.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Ground State Configuration The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The electron configuration of bromine. Bromine Ground State Configuration.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Ground State Configuration Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The complete electron configuration of the bromine is: The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron. Bromine Ground State Configuration.
From www.chegg.com
Solved Write the electron configuration for each element in Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. From the electrons. Bromine Ground State Configuration.
From www.numerade.com
SOLVED Write groundstate electron configurations for the following Bromine Ground State Configuration The complete electron configuration of the bromine is: The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. From the electrons in an atom, to the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 1s 2 2s. Bromine Ground State Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Bromine Ground State Configuration Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. From the electrons in an atom, to the. From the electrons in an atom, to the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron configurations are the foundation for understanding molecular bonding,. Bromine Ground State Configuration.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. From the electrons in an atom, to the. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The complete electron configuration of the. Bromine Ground State Configuration.
From www.slideserve.com
PPT Atom video PowerPoint Presentation ID386307 Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding,. Bromine Ground State Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Ground State Configuration From the electrons in an atom, to the. The complete electron configuration of the bromine is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral. Bromine Ground State Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Ground State Configuration From the electrons in an atom, to the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The way we designate electronic configurations for cations. Bromine Ground State Configuration.
From www.chegg.com
Solved BA 1A 2A 3A 4A 5A 6A 7A He Sb Te Xe Pa (1) What is Bromine Ground State Configuration Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. From the electrons in an atom, to the. The ground state. Bromine Ground State Configuration.
From www.webelements.com
Elements Periodic Table » Bromine » properties of free atoms Bromine Ground State Configuration The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The electron configuration of bromine is. Bromine Ground State Configuration.
From exonchlhy.blob.core.windows.net
What Is The Complete Ground State Electron Configuration For The Bromine Ground State Configuration This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The electron. Bromine Ground State Configuration.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Ground State Configuration This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar. Bromine Ground State Configuration.
From exonchlhy.blob.core.windows.net
What Is The Complete Ground State Electron Configuration For The Bromine Ground State Configuration Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The complete electron configuration of the bromine is: Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electron configuration. Bromine Ground State Configuration.
From www.youtube.com
Br electronic configurationHow to write electronic configuration of Bromine Ground State Configuration From the electrons in an atom, to the. From the electrons in an atom, to the. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The complete electron configuration of the bromine is: The electron configuration of bromine is. Bromine Ground State Configuration.
From geteducationskills.com
Ground State Electron Configuration An Overview Get Education Bromine Ground State Configuration From the electrons in an atom, to the. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The ground state electron configuration of ground state gaseous neutral bromine is [ar].3d 10.4s 2.4p 5. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: Ground state electron configurations are. Bromine Ground State Configuration.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Ground State Configuration The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. From the electrons in an atom, to the. The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. The complete electron configuration of the bromine is: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.. Bromine Ground State Configuration.
From studylib.net
AP Chemistry Ch 7,2,3 Bromine Ground State Configuration From the electrons in an atom, to the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The complete electron configuration of. Bromine Ground State Configuration.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Ground State Configuration The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The complete electron configuration of the bromine is: From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. The ground state electron configuration of ground. Bromine Ground State Configuration.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Ground State Configuration The way we designate electronic configurations for cations and anions is essentially similar to that for neutral atoms in their ground state. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. From the electrons in an atom, to the. Ground state electron configurations are the foundation for understanding molecular bonding, properties, and structures. Ground state. Bromine Ground State Configuration.