Titrating Hcl With Naoh Calculations . For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Hcl and naoh reacts in 1:1 ratio (in same amount). To decide required amount (mol) and volume, the. Multiply the molarity of the strong base naoh by the volume of. \ce{naoh}\) is required to neutralize. Important factors and equations of hcl + naoh reaction and its titration curve.
from www.thinkswap.com
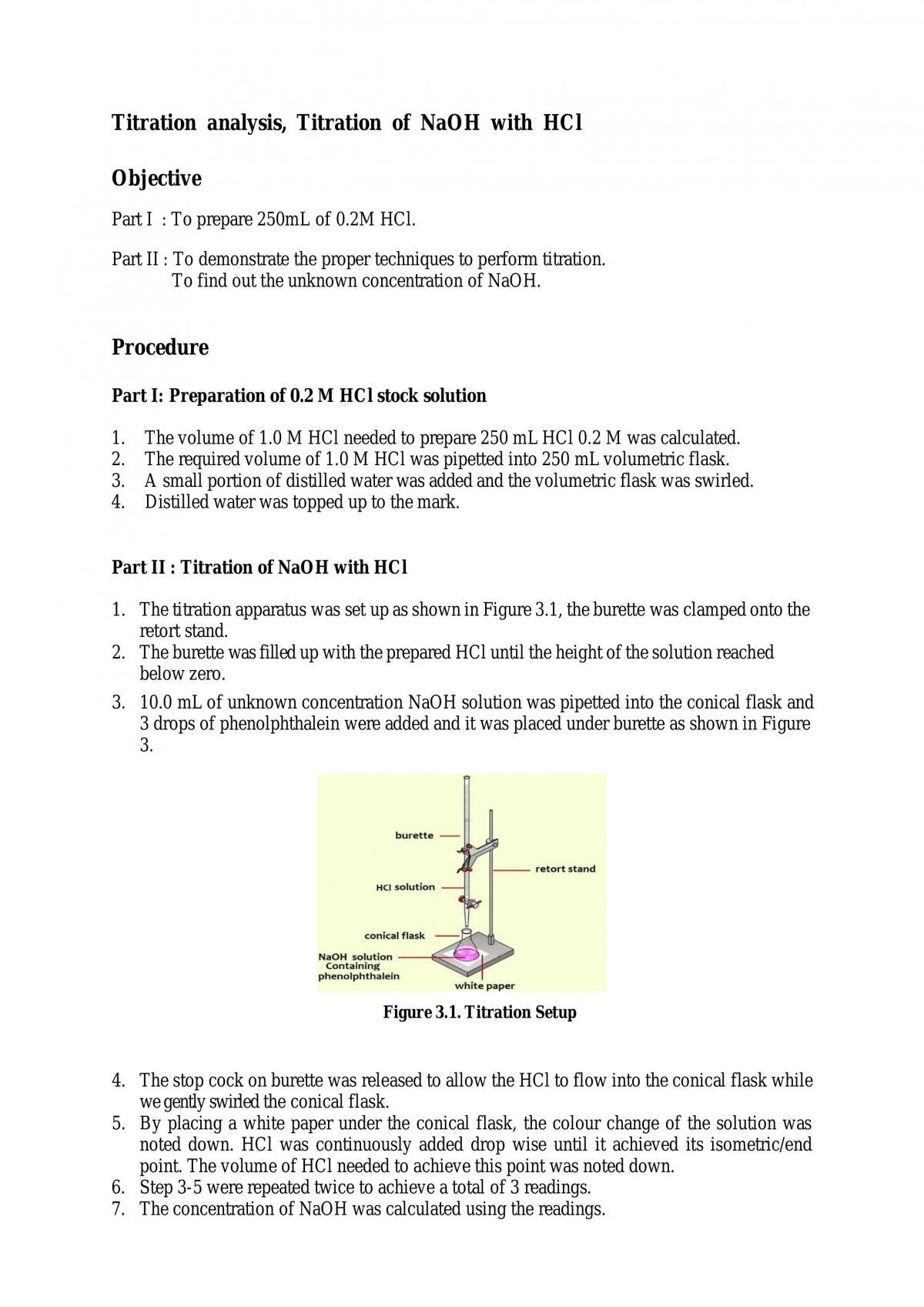
A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to neutralize. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Multiply the molarity of the strong base naoh by the volume of. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Important factors and equations of hcl + naoh reaction and its titration curve. Hcl and naoh reacts in 1:1 ratio (in same amount). To decide required amount (mol) and volume, the.
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General
Titrating Hcl With Naoh Calculations To decide required amount (mol) and volume, the. \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hcl and naoh reacts in 1:1 ratio (in same amount). A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. To decide required amount (mol) and volume, the. Multiply the molarity of the strong base naoh by the volume of. Important factors and equations of hcl + naoh reaction and its titration curve. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titrating Hcl With Naoh Calculations A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED In a titration of hydrochloric acid against sodium hydroxide Titrating Hcl With Naoh Calculations To decide required amount (mol) and volume, the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the strong base naoh by the volume of. Important factors and equations of hcl + naoh reaction and its titration curve. For a neutralization reaction with a. Titrating Hcl With Naoh Calculations.
From mungfali.com
HCl NaOH Titration Titrating Hcl With Naoh Calculations For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Hcl and naoh reacts in 1:1 ratio (in same amount). Important factors and equations of hcl + naoh reaction and its titration curve. Multiply the molarity of the strong base naoh by the volume of. A titration is used to find an unknown. Titrating Hcl With Naoh Calculations.
From www.chegg.com
Attached is the titration curve of NaOH titrating HCl Titrating Hcl With Naoh Calculations A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. \ce{naoh}\) is required to neutralize. Hcl and naoh reacts in 1:1 ratio (in same amount). For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at. Titrating Hcl With Naoh Calculations.
From byjus.com
8. Calculate the weight of NaOH NaHCO3 whose 50ml each is completely Titrating Hcl With Naoh Calculations A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the strong base naoh by the volume of. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Hcl and naoh reacts in 1:1 ratio (in same amount).. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED 'Determination the normality of a sodium hydroxide solution Titrating Hcl With Naoh Calculations Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh reacts in 1:1 ratio (in same amount). Important factors and equations of hcl + naoh reaction and its titration curve. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration of. Titrating Hcl With Naoh Calculations.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titrating Hcl With Naoh Calculations To decide required amount (mol) and volume, the. \ce{naoh}\) is required to neutralize. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Titrating Hcl With Naoh Calculations.
From www.chegg.com
Solved 1. Titrating HCl solution with NaOH solution 1. Titrating Hcl With Naoh Calculations Multiply the molarity of the strong base naoh by the volume of. Important factors and equations of hcl + naoh reaction and its titration curve. \ce{naoh}\) is required to neutralize. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is carried out for 25.00 ml of. Titrating Hcl With Naoh Calculations.
From mungfali.com
HCl NaOH Titration Titrating Hcl With Naoh Calculations \ce{naoh}\) is required to neutralize. Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh reacts in 1:1 ratio (in same amount). A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. For a neutralization reaction. Titrating Hcl With Naoh Calculations.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titrating Hcl With Naoh Calculations Hcl and naoh reacts in 1:1 ratio (in same amount). Multiply the molarity of the strong base naoh by the volume of. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. To decide required amount (mol) and. Titrating Hcl With Naoh Calculations.
From www.slideserve.com
PPT Reactions in Aqueous Solutions Calculations PowerPoint Titrating Hcl With Naoh Calculations A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Important factors and equations of hcl + naoh reaction and its titration curve. Hcl and naoh reacts in 1:1 ratio (in same amount). In a titration of sulfuric acid against sodium. Titrating Hcl With Naoh Calculations.
From www.slideserve.com
PPT Volumetric Calculations PowerPoint Presentation, free download Titrating Hcl With Naoh Calculations For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Hcl and naoh reacts in 1:1 ratio (in same amount). A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to neutralize. Multiply the molarity of the strong base. Titrating Hcl With Naoh Calculations.
From mungfali.com
Acid Base Titration Calculation Titrating Hcl With Naoh Calculations In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: To decide required amount (mol) and volume, the. Multiply the molarity of the strong base naoh by the volume of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure.. Titrating Hcl With Naoh Calculations.
From mungfali.com
HCl NaOH Titration Titrating Hcl With Naoh Calculations A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known. Titrating Hcl With Naoh Calculations.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Titrating Hcl With Naoh Calculations In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Multiply the molarity of the strong base naoh by the volume of. Hcl and naoh reacts in 1:1 ratio (in same amount). To decide required amount (mol) and volume, the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of. Titrating Hcl With Naoh Calculations.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Titrating Hcl With Naoh Calculations To decide required amount (mol) and volume, the. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Important factors and equations of hcl + naoh reaction and its titration curve. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh. Titrating Hcl With Naoh Calculations.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titrating Hcl With Naoh Calculations \ce{naoh}\) is required to neutralize. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the. Titrating Hcl With Naoh Calculations.
From mungfali.com
HCl NaOH Titration Titrating Hcl With Naoh Calculations For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Multiply the molarity of the strong base naoh by the volume of. Important factors and equations of hcl + naoh reaction and its titration curve. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of. Titrating Hcl With Naoh Calculations.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titrating Hcl With Naoh Calculations \ce{naoh}\) is required to neutralize. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. To decide required amount (mol) and volume, the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hcl and naoh reacts in 1:1 ratio (in same amount). Important factors and equations of. Titrating Hcl With Naoh Calculations.
From www.chegg.com
Solved II. Titrating HCl Solution with NaOH Solution first Titrating Hcl With Naoh Calculations In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hcl and naoh reacts in 1:1 ratio (in same amount). Important factors and equations of hcl + naoh reaction and its titration curve. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Multiply the molarity of the. Titrating Hcl With Naoh Calculations.
From www.chegg.com
Solved HCl(aq) + NaOH(aq)) NaCl(aq) + H2O(1) From the data Titrating Hcl With Naoh Calculations For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Hcl and naoh reacts in 1:1 ratio (in same amount). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Important factors and equations of hcl + naoh reaction and its titration curve. A titration is used to find an unknown concentration. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED Consider the model of titrating hydrochloric acid in the given Titrating Hcl With Naoh Calculations Important factors and equations of hcl + naoh reaction and its titration curve. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. \ce{naoh}\) is required to neutralize. To decide. Titrating Hcl With Naoh Calculations.
From www.coursehero.com
[Solved] Titrating HCL solution with NaOH solutions. Please fill this Titrating Hcl With Naoh Calculations \ce{naoh}\) is required to neutralize. Multiply the molarity of the strong base naoh by the volume of. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is carried out for 25.00 ml of 0.100 m. Titrating Hcl With Naoh Calculations.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titrating Hcl With Naoh Calculations Multiply the molarity of the strong base naoh by the volume of. \ce{naoh}\) is required to neutralize. Important factors and equations of hcl + naoh reaction and its titration curve. To decide required amount (mol) and volume, the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hcl and naoh reacts in 1:1 ratio (in same amount). A. Titrating Hcl With Naoh Calculations.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titrating Hcl With Naoh Calculations Important factors and equations of hcl + naoh reaction and its titration curve. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Hcl and naoh reacts in 1:1 ratio. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate Titrating Hcl With Naoh Calculations For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. Important factors and equations of hcl + naoh reaction and its titration curve. Hcl. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVEDCalculate the volume in milliliters of a 1.420 M NaOH solution Titrating Hcl With Naoh Calculations Multiply the molarity of the strong base naoh by the volume of. Important factors and equations of hcl + naoh reaction and its titration curve. Hcl and naoh reacts in 1:1 ratio (in same amount). For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. \ce{naoh}\) is required to neutralize. To decide required. Titrating Hcl With Naoh Calculations.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titrating Hcl With Naoh Calculations Important factors and equations of hcl + naoh reaction and its titration curve. To decide required amount (mol) and volume, the. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. \ce{naoh}\). Titrating Hcl With Naoh Calculations.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Titrating Hcl With Naoh Calculations In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: \ce{naoh}\) is required to neutralize. Important factors and equations of hcl + naoh reaction and its titration curve. Hcl and naoh reacts in 1:1 ratio (in same amount). Multiply the molarity of the strong base naoh by the volume of. For a neutralization reaction with a 1:1 stoichiometric ratio. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED A 40 mL solution of .750 M acetic acid (Ka=1.76x10^5) is Titrating Hcl With Naoh Calculations Hcl and naoh reacts in 1:1 ratio (in same amount). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to neutralize. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Titrating Hcl With Naoh Calculations.
From mungfali.com
HCl NaOH Titration Titrating Hcl With Naoh Calculations Hcl and naoh reacts in 1:1 ratio (in same amount). For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. \ce{naoh}\) is required to neutralize. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. In a titration of sulfuric acid against. Titrating Hcl With Naoh Calculations.
From www.numerade.com
SOLVED Given this balanced chemical equation and the following Titrating Hcl With Naoh Calculations Hcl and naoh reacts in 1:1 ratio (in same amount). \ce{naoh}\) is required to neutralize. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Important factors and equations of hcl + naoh reaction and its titration curve. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \:. Titrating Hcl With Naoh Calculations.
From studylib.net
Titration of HCl with NaOH Titrating Hcl With Naoh Calculations Important factors and equations of hcl + naoh reaction and its titration curve. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Multiply the molarity of the strong base naoh by the volume of. A titration is used to find an. Titrating Hcl With Naoh Calculations.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titrating Hcl With Naoh Calculations \ce{naoh}\) is required to neutralize. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Multiply the molarity of the strong base naoh by the volume of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is. Titrating Hcl With Naoh Calculations.
From www.vrogue.co
Titration Practical And Calculation Naoh And Hcl Yout vrogue.co Titrating Hcl With Naoh Calculations In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. To decide required. Titrating Hcl With Naoh Calculations.