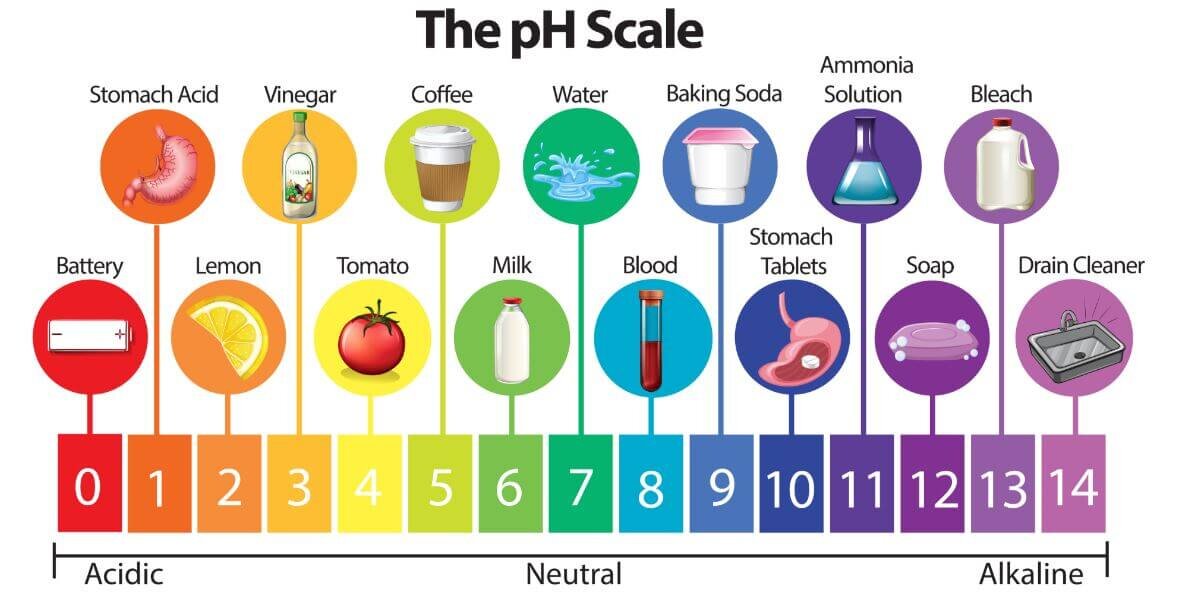
Baking Soda Is Acid Or Base . Learn why baking soda is a base and how it can be used for various purposes. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Discover how it reacts with acids, how to use it. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Learn more about its chemical composition, properties, and applications. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. When heated, baking soda breaks. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Find out the answers to frequently asked questions about baking soda. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9.
from www.lewislatimerhouse.org
Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn why baking soda is a base and how it can be used for various purposes. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. When heated, baking soda breaks. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Find out the answers to frequently asked questions about baking soda. Discover how it reacts with acids, how to use it.
Baking Soda Experiments — Lewis Latimer House Museum
Baking Soda Is Acid Or Base When heated, baking soda breaks. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn more about its chemical composition, properties, and applications. When heated, baking soda breaks. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Discover how it reacts with acids, how to use it. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Find out the answers to frequently asked questions about baking soda. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn why baking soda is a base and how it can be used for various purposes. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications.
From www.quora.com
How acidic is baking soda? Quora Baking Soda Is Acid Or Base Find out the answers to frequently asked questions about baking soda. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn more about its chemical composition, properties, and applications. Discover how it reacts with acids, how to use it. Baking soda or sodium bicarbonate. Baking Soda Is Acid Or Base.
From sciencenotes.org
Household Acids and Bases Baking Soda Is Acid Or Base When heated, baking soda breaks. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn more about its chemical composition, properties, and applications. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Baking. Baking Soda Is Acid Or Base.
From slideplayer.com
ACIDS AND BASES ppt download Baking Soda Is Acid Or Base Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Baking soda (nahco3) is basic because it dissociates into sodium ions and. Baking Soda Is Acid Or Base.
From sciencenotes.org
Facts About Acids and Bases Baking Soda Is Acid Or Base Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn why baking soda is a base and how it can be used for various purposes. When heated, baking soda breaks. Find out the answers to frequently asked questions about baking soda. Yes, although baking soda is primarily a base, it can also exhibit acidic. Baking Soda Is Acid Or Base.
From www.breadnewbie.com
Is Baking Soda an Acid or a Base? (It's a Base) Baking Soda Is Acid Or Base Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Find out the answers to frequently asked questions about baking soda. When heated, baking soda breaks. Learn how baking soda is amphoteric, how it differs from baking. Baking Soda Is Acid Or Base.
From architecturefor.weebly.com
What is the difference between baking soda and baking powder Baking Soda Is Acid Or Base It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Find out the answers to frequently asked questions about baking soda. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of. Baking Soda Is Acid Or Base.
From www.worksheetsplanet.com
Acid and Bases Differences Baking Soda Is Acid Or Base Discover how it reacts with acids, how to use it. Learn more about its chemical composition, properties, and applications. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of. Baking Soda Is Acid Or Base.
From www.youtube.com
Acids bases and salts baking soda its uses and preparation class 10 Baking Soda Is Acid Or Base Learn more about its chemical composition, properties, and applications. Find out the answers to frequently asked questions about baking soda. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. When heated, baking soda breaks. Learn why. Baking Soda Is Acid Or Base.
From sciencenotes.org
The pH Scale of Common Chemicals Baking Soda Is Acid Or Base When heated, baking soda breaks. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Learn more about its chemical composition, properties, and applications. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn how baking soda. Baking Soda Is Acid Or Base.
From www.learnatnoon.com
What are the differences between Acids and Bases? Noon Baking Soda Is Acid Or Base Learn more about its chemical composition, properties, and applications. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Discover how it reacts with acids, how to. Baking Soda Is Acid Or Base.
From sciencenotes.org
AcidBase Chemistry Baking Soda Is Acid Or Base Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Find out the answers to frequently asked questions about baking soda. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn more about its chemical composition, properties, and applications. It can neutralize acids, dissolve in water,. Baking Soda Is Acid Or Base.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Baking Soda Is Acid Or Base Find out the answers to frequently asked questions about baking soda. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Discover how it reacts with acids, how to use it. Baking. Baking Soda Is Acid Or Base.
From acidbaseeducation.weebly.com
Common Household Items acid & Base Education Baking Soda Is Acid Or Base Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Find out the answers to frequently asked questions about baking soda. Discover how it reacts with acids, how to use it. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Baking soda (nahco3) is basic because. Baking Soda Is Acid Or Base.
From scienceandsf.com
Acids, Bases and pH. Scienceandsf A Blog Published by Robert A. Lawler Baking Soda Is Acid Or Base When heated, baking soda breaks. Discover how it reacts with acids, how to use it. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Find out the answers to frequently asked questions about baking soda. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain. Baking Soda Is Acid Or Base.
From howchimp.com
Is Baking Soda an Acid or Base? HowChimp Baking Soda Is Acid Or Base It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Learn more about its chemical composition, properties, and applications. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form. Baking Soda Is Acid Or Base.
From howchimp.com
Is Baking Soda an Acid or Base? HowChimp Baking Soda Is Acid Or Base It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Discover how it reacts with acids, how to use it. Find out the answers to frequently asked questions about baking soda. Learn more about its chemical. Baking Soda Is Acid Or Base.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable Baking Soda Is Acid Or Base Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. When heated, baking soda breaks. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. It can neutralize acids, dissolve in water, and react with acids to produce. Baking Soda Is Acid Or Base.
From kate-has-abbott.blogspot.com
Is Baking Soda an Acid or Base KatehasAbbott Baking Soda Is Acid Or Base Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Learn why baking soda is a base and how. Baking Soda Is Acid Or Base.
From www.lewislatimerhouse.org
Baking Soda Experiments — Lewis Latimer House Museum Baking Soda Is Acid Or Base Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Discover how it reacts with acids, how to. Baking Soda Is Acid Or Base.
From slideplayer.com
Acids and Bases. ppt video online download Baking Soda Is Acid Or Base Learn more about its chemical composition, properties, and applications. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Find out the answers to frequently asked questions about baking soda. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn how baking soda is amphoteric, how it differs. Baking Soda Is Acid Or Base.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary Baking Soda Is Acid Or Base Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. When heated, baking soda breaks. Baking soda or sodium bicarbonate is a base with a ph of 8. Baking Soda Is Acid Or Base.
From thisonevsthatone.com
Baking Soda vs Baking Powder What's the difference? Baking Soda Is Acid Or Base Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Find out the answers to frequently asked questions about baking soda. Yes, although baking soda is primarily a base, it can also. Baking Soda Is Acid Or Base.
From www.youtube.com
Baking Soda Acids Bases and Salts Chemistry Science Class 10 Baking Soda Is Acid Or Base Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Discover how it reacts with acids, how to use it. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Learn how baking soda is amphoteric, how it differs. Baking Soda Is Acid Or Base.
From www.youtube.com
Baking soda Acids bases and salts class 10 2D Content YouTube Baking Soda Is Acid Or Base Discover how it reacts with acids, how to use it. When heated, baking soda breaks. Find out the answers to frequently asked questions about baking soda. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react. Baking Soda Is Acid Or Base.
From www.youtube.com
BAKING SODA(ACIDS, BASES AND SAITS) YouTube Baking Soda Is Acid Or Base Learn more about its chemical composition, properties, and applications. When heated, baking soda breaks. Find out the answers to frequently asked questions about baking soda. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used. Baking Soda Is Acid Or Base.
From slideplayer.com
Acids and Bases Lesson ppt download Baking Soda Is Acid Or Base Learn why baking soda is a base and how it can be used for various purposes. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Discover how it reacts with acids, how to use it. Learn why baking soda, also known as sodium bicarbonate, is a base with a. Baking Soda Is Acid Or Base.
From courses.lumenlearning.com
Discussion Acids and Bases at Home Chemistry for Majors Baking Soda Is Acid Or Base Find out the answers to frequently asked questions about baking soda. When heated, baking soda breaks. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Discover how it reacts with acids, how to use it. Learn. Baking Soda Is Acid Or Base.
From www.breadnewbie.com
Is Baking Soda an Acid or a Base? (It's a Base) Baking Soda Is Acid Or Base Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn more about its chemical composition, properties, and applications. Discover how it reacts with acids, how to use it. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. When heated, baking. Baking Soda Is Acid Or Base.
From www.youtube.com
Baking Soda Sodium Hydrogen Carbonate Acids Bases and Salts Part Baking Soda Is Acid Or Base Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under certain conditions. Learn more about its chemical composition, properties, and applications. Learn why baking soda is a base and how it can be used for various purposes.. Baking Soda Is Acid Or Base.
From printables.it.com
Baking Soda Science Printables Chemical Reaction Free Printable Download Baking Soda Is Acid Or Base When heated, baking soda breaks. Find out the answers to frequently asked questions about baking soda. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Discover. Baking Soda Is Acid Or Base.
From katierossparks.blogspot.com
Baking Soda Chemical Formula KatierosSparks Baking Soda Is Acid Or Base Learn more about its chemical composition, properties, and applications. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Find out the answers to frequently asked questions about baking soda. Learn why. Baking Soda Is Acid Or Base.
From www.youtube.com
Baking Soda Acids Bases and Salts Chapter 2 (L11) Class 10 Baking Soda Is Acid Or Base Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Yes, although baking soda is primarily a base, it can also exhibit acidic properties under. Baking Soda Is Acid Or Base.
From slideplayer.com
ACIDS AND BASES. ppt download Baking Soda Is Acid Or Base Baking soda or sodium bicarbonate is a base with a ph of 8 to 9. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used in various applications. Learn more about its chemical composition, properties, and applications. Learn why baking soda is a base and how it can be used for various purposes.. Baking Soda Is Acid Or Base.
From www.pinterest.ca
A Simple Explanation the Difference Between Baking Soda & Baking Baking Soda Is Acid Or Base Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to form hydroxide ions and carbonic acid. Learn why baking soda, also known as sodium bicarbonate, is a base with a ph of around 9. Learn how baking soda is amphoteric, how it differs from baking powder, and how it is used. Baking Soda Is Acid Or Base.
From cakedecorist.com
Is Soda An Acid Or Base? Cake Decorist Baking Soda Is Acid Or Base Find out the answers to frequently asked questions about baking soda. Learn more about its chemical composition, properties, and applications. When heated, baking soda breaks. It can neutralize acids, dissolve in water, and react with acids to produce carbon dioxide gas. Baking soda (nahco3) is basic because it dissociates into sodium ions and bicarbonate ions, which react with water to. Baking Soda Is Acid Or Base.