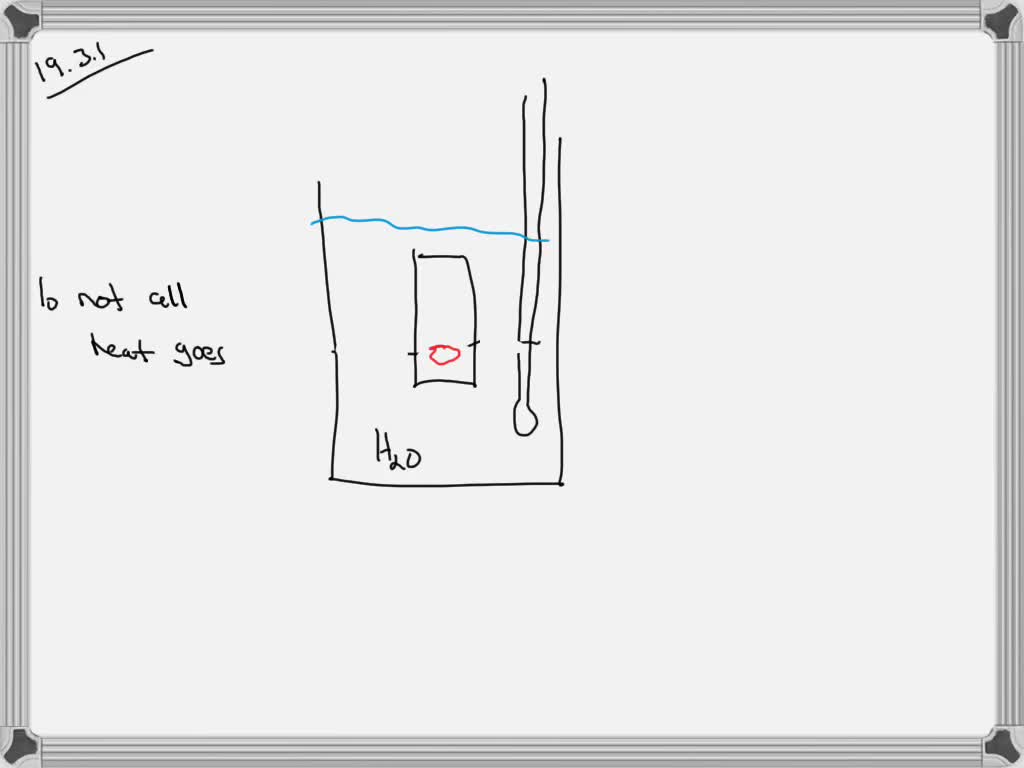
Sources Of Error In A Calorimetry Lab . To apply these δh values in a hess’s law calculation to determine the enthalpy of. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. A simple explanation of the limitations. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Use your calculated average deviation and relative error in your discussion. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. This can be reduced by insulating the sides of the calorimeter and adding a lid.
from www.numerade.com
Use your calculated average deviation and relative error in your discussion. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. This can be reduced by insulating the sides of the calorimeter and adding a lid. To apply these δh values in a hess’s law calculation to determine the enthalpy of. A simple explanation of the limitations. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings.
Name three possible sources of error in a calorimetry experiment
Sources Of Error In A Calorimetry Lab A simple explanation of the limitations. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. Use your calculated average deviation and relative error in your discussion. This can be reduced by insulating the sides of the calorimeter and adding a lid. A simple explanation of the limitations. To apply these δh values in a hess’s law calculation to determine the enthalpy of. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry.
From www.scribd.com
EXPERIMENT 302 Heat and Calorimetry Analysis Sources of Error PDF Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To apply these δh values in a hess’s law calculation to determine the enthalpy of. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. This can be reduced by insulating the sides of the calorimeter and adding a lid. A. Sources Of Error In A Calorimetry Lab.
From www.studocu.com
Calorimetry LAB Question 1 1. In part I of the experiment, what is a Sources Of Error In A Calorimetry Lab To apply these δh values in a hess’s law calculation to determine the enthalpy of. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To experimentally. Sources Of Error In A Calorimetry Lab.
From www.slideserve.com
PPT Chapter 21 Measurement Analysis PowerPoint Presentation, free Sources Of Error In A Calorimetry Lab This can be reduced by insulating the sides of the calorimeter and adding a lid. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. A simple explanation of the limitations. (an average deviation of 0.02 j/g°c and a relative error of. Sources Of Error In A Calorimetry Lab.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To apply these δh values in a hess’s law calculation to determine the enthalpy of. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it. Sources Of Error In A Calorimetry Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Sources Of Error In A Calorimetry Lab This can be reduced by insulating the sides of the calorimeter and adding a lid. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To apply these δh values in a hess’s law calculation to determine the enthalpy of. Use your. Sources Of Error In A Calorimetry Lab.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Sources Of Error In A Calorimetry Lab A simple explanation of the limitations. To apply these δh values in a hess’s law calculation to determine the enthalpy of. Use your calculated average deviation and relative error in your discussion. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. This can be reduced by insulating the sides of the calorimeter and. Sources Of Error In A Calorimetry Lab.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. A simple explanation of the limitations. To apply these δh values in a hess’s law calculation to determine the enthalpy of. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer.. Sources Of Error In A Calorimetry Lab.
From studylib.net
5.1 b) CALORIMETRY Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To apply these δh values in a hess’s law calculation to determine the enthalpy of. Use your. Sources Of Error In A Calorimetry Lab.
From www.chegg.com
Bomb Calorimetry CHEM 3625 Prelab Section 1 When a Sources Of Error In A Calorimetry Lab One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. To apply. Sources Of Error In A Calorimetry Lab.
From studylib.net
Lab Calorimetry High School Science Help Sources Of Error In A Calorimetry Lab This can be reduced by insulating the sides of the calorimeter and adding a lid. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. A simple explanation of the limitations. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in. Sources Of Error In A Calorimetry Lab.
From studylib.net
Calorimetry Lab Report Problem To experimentally determine the Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that. Sources Of Error In A Calorimetry Lab.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. This can be reduced by insulating the sides of the calorimeter and adding a lid. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To apply these δh values in. Sources Of Error In A Calorimetry Lab.
From sincerenewsknight.blogspot.com
Types of Errors in Measurement Sources Of Error In A Calorimetry Lab A simple explanation of the limitations. To apply these δh values in a hess’s law calculation to determine the enthalpy of. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. One example of a systematic error could be using. Sources Of Error In A Calorimetry Lab.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 Sources Of Error In A Calorimetry Lab To apply these δh values in a hess’s law calculation to determine the enthalpy of. Use your calculated average deviation and relative error in your discussion. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To experimentally measure the δh values. Sources Of Error In A Calorimetry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Sources Of Error In A Calorimetry Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply these δh values in a hess’s law calculation to determine the enthalpy of. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. A simple explanation of the limitations. Use your calculated average deviation and relative error. Sources Of Error In A Calorimetry Lab.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. A simple explanation of the limitations. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. This can be reduced by insulating the sides of the calorimeter and adding a lid. (an average deviation of 0.02 j/g°c and a relative error of less than. Sources Of Error In A Calorimetry Lab.
From www.chegg.com
Solved Lab Calorimetry of a salt solution Procedure Sources Of Error In A Calorimetry Lab To apply these δh values in a hess’s law calculation to determine the enthalpy of. A simple explanation of the limitations. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. This can be reduced by insulating the sides of the calorimeter and adding a lid. The biggest source of error in calorimetry is usually unwanted. Sources Of Error In A Calorimetry Lab.
From klaqayslh.blob.core.windows.net
Calorimeter Experiment Sources Of Error at Rudy Reichman blog Sources Of Error In A Calorimetry Lab (an average deviation of 0.02 j/g°c and a relative error of less than 10%. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply. Sources Of Error In A Calorimetry Lab.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Sources Of Error In A Calorimetry Lab This can be reduced by insulating the sides of the calorimeter and adding a lid. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry.. Sources Of Error In A Calorimetry Lab.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. A simple explanation of the limitations. Use your calculated average deviation and relative error in your discussion. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. This can be reduced by insulating the sides of the calorimeter and adding a. Sources Of Error In A Calorimetry Lab.
From dxosivrpk.blob.core.windows.net
Food Calorimetry Lab Procedure at Ralph Safford blog Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Use. Sources Of Error In A Calorimetry Lab.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Sources Of Error In A Calorimetry Lab A simple explanation of the limitations. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To apply these δh values in a hess’s law calculation to determine the enthalpy of. Use your calculated average deviation and relative error in your discussion. To experimentally measure the δh values of two reactions using the technique of. Sources Of Error In A Calorimetry Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Sources Of Error In A Calorimetry Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. A simple explanation of the limitations. Use your calculated average deviation and relative error in your. Sources Of Error In A Calorimetry Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Sources Of Error In A Calorimetry Lab Use your calculated average deviation and relative error in your discussion. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. This can be reduced by insulating the sides of the calorimeter and adding a lid. To apply these δh values in a hess’s law calculation to determine the enthalpy of. The biggest source. Sources Of Error In A Calorimetry Lab.
From www.youtube.com
R1.1.4 What are the sources of error in calorimetry? YouTube Sources Of Error In A Calorimetry Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. To apply these δh values in a hess’s law calculation to determine the enthalpy of. One example of a systematic error could be using a ph meter that is incorrectly. Sources Of Error In A Calorimetry Lab.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Sources Of Error In A Calorimetry Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. A simple explanation of the limitations. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. This can be reduced by insulating the sides of the. Sources Of Error In A Calorimetry Lab.
From www.slideshare.net
4 Calorimetry Sources Of Error In A Calorimetry Lab One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. To apply these. Sources Of Error In A Calorimetry Lab.
From www.youtube.com
Effects of experimental errors on enthalpy measurements YouTube Sources Of Error In A Calorimetry Lab (an average deviation of 0.02 j/g°c and a relative error of less than 10%. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. This can be. Sources Of Error In A Calorimetry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Sources Of Error In A Calorimetry Lab To apply these δh values in a hess’s law calculation to determine the enthalpy of. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. One example of a systematic error could be using a ph meter that is incorrectly calibrated,. Sources Of Error In A Calorimetry Lab.
From www.numerade.com
Name three possible sources of error in a calorimetry experiment Sources Of Error In A Calorimetry Lab This can be reduced by insulating the sides of the calorimeter and adding a lid. Use your calculated average deviation and relative error in your discussion. To apply these δh values in a hess’s law calculation to determine the enthalpy of. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. The biggest source of error. Sources Of Error In A Calorimetry Lab.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. Use your calculated average deviation and relative error in your discussion. To apply these δh values in a hess’s law calculation to determine the enthalpy of. This can be reduced by. Sources Of Error In A Calorimetry Lab.
From dxozhmrqx.blob.core.windows.net
Error Identification Examples at Geraldine Bryant blog Sources Of Error In A Calorimetry Lab To apply these δh values in a hess’s law calculation to determine the enthalpy of. A simple explanation of the limitations. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Use your calculated average deviation and relative error in your discussion.. Sources Of Error In A Calorimetry Lab.
From studylib.net
STUDY GUIDE Osmosis/Diffusion, Measurement, Source of Error Sources Of Error In A Calorimetry Lab To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. A simple explanation of the limitations. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed. Sources Of Error In A Calorimetry Lab.
From dxosivrpk.blob.core.windows.net
Food Calorimetry Lab Procedure at Ralph Safford blog Sources Of Error In A Calorimetry Lab (an average deviation of 0.02 j/g°c and a relative error of less than 10%. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. A simple explanation of the limitations. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply these δh values in a hess’s law. Sources Of Error In A Calorimetry Lab.
From www.numerade.com
SOLVED What are two sources of error in Thermochemistry and Specific Sources Of Error In A Calorimetry Lab The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Use your calculated average deviation and relative error in your discussion. This can be reduced by insulating the sides of the calorimeter and adding a lid. (an average deviation of 0.02 j/g°c and a relative error of less than 10%. A simple explanation of the. Sources Of Error In A Calorimetry Lab.