Calculate Kb For The Phenolate Ion . The numerical value of ka and kb can be determined from an experiment. For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). We need to find the kb of format iron. \[ k_b = \frac{k_w}{k_a} \] K b = k w k a. The k acid dissociation constant of formic acid is more than 10 to the four negative four. Which base in table 14.2 ( sec. Your solution’s ready to go! Similarly, the equilibrium constant for the reaction of a. Calculating k a and k b. A solution of known concentration is prepared and its ph is measured with an. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. To find \( k_b \) for the phenolate ion, we rearrange the equation:
from www.chegg.com
Which base in table 14.2 ( sec. The numerical value of ka and kb can be determined from an experiment. We need to find the kb of format iron. The k acid dissociation constant of formic acid is more than 10 to the four negative four. K b = k w k a. Your solution’s ready to go! We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Similarly, the equilibrium constant for the reaction of a. Calculating k a and k b. A solution of known concentration is prepared and its ph is measured with an.
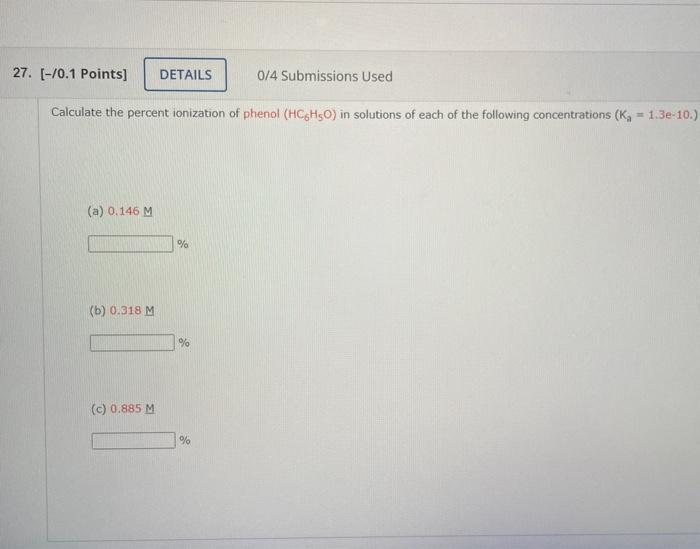
Solved Calculate the percent ionization of phenol (HC6H5O)
Calculate Kb For The Phenolate Ion Your solution’s ready to go! Which base in table 14.2 ( sec. The k acid dissociation constant of formic acid is more than 10 to the four negative four. To find \( k_b \) for the phenolate ion, we rearrange the equation: Calculating k a and k b. \[ k_b = \frac{k_w}{k_a} \] A solution of known concentration is prepared and its ph is measured with an. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. The numerical value of ka and kb can be determined from an experiment. We need to find the kb of format iron. Your solution’s ready to go! K b = k w k a. For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). Similarly, the equilibrium constant for the reaction of a.
From www.numerade.com
Calculate the pH of 0.100 M sodium phenolate, C6H5ONa, the sodium salt Calculate Kb For The Phenolate Ion To find \( k_b \) for the phenolate ion, we rearrange the equation: The k acid dissociation constant of formic acid is more than 10 to the four negative four. A solution of known concentration is prepared and its ph is measured with an. Your solution’s ready to go! \[ k_b = \frac{k_w}{k_a} \] For an aqueous solution of a. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Calculate the percent ionization of phenol (HC6H50) Calculate Kb For The Phenolate Ion \[ k_b = \frac{k_w}{k_a} \] Similarly, the equilibrium constant for the reaction of a. The numerical value of ka and kb can be determined from an experiment. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Calculating k a and k b. Your solution’s ready. Calculate Kb For The Phenolate Ion.
From chem.ucalgary.ca
Chapter 24 Phenols Calculate Kb For The Phenolate Ion We need to find the kb of format iron. \[ k_b = \frac{k_w}{k_a} \] We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Similarly, the equilibrium constant for the reaction of a. For an aqueous solution of a weak acid, the dissociation constant is called. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVED Phenol (C6H6OH) has a Ka of 1.3 x 10^10. Write out the Ka Calculate Kb For The Phenolate Ion K b = k w k a. To find \( k_b \) for the phenolate ion, we rearrange the equation: A solution of known concentration is prepared and its ph is measured with an. Your solution’s ready to go! Which base in table 14.2 ( sec. We need to find the kb of format iron. The numerical value of ka. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Find the concentration of phenol and phenolate ion in Calculate Kb For The Phenolate Ion K b = k w k a. The numerical value of ka and kb can be determined from an experiment. A solution of known concentration is prepared and its ph is measured with an. The k acid dissociation constant of formic acid is more than 10 to the four negative four. We need to find the kb of format iron.. Calculate Kb For The Phenolate Ion.
From slidetodoc.com
Phenol Nomenclature Acidity substitution effect on the acidity Calculate Kb For The Phenolate Ion Which base in table 14.2 ( sec. A solution of known concentration is prepared and its ph is measured with an. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. K b = k w k a. We need to find the kb of format. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion A solution of known concentration is prepared and its ph is measured with an. Which base in table 14.2 ( sec. Similarly, the equilibrium constant for the reaction of a. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. The numerical value of ka and. Calculate Kb For The Phenolate Ion.
From pharmacyscope.com
Phenols Pharmacy Scope Calculate Kb For The Phenolate Ion Which base in table 14.2 ( sec. K b = k w k a. The k acid dissociation constant of formic acid is more than 10 to the four negative four. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Your solution’s ready to go!. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion \[ k_b = \frac{k_w}{k_a} \] A solution of known concentration is prepared and its ph is measured with an. Which base in table 14.2 ( sec. The numerical value of ka and kb can be determined from an experiment. We need to find the kb of format iron. Your solution’s ready to go! To find \( k_b \) for the. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Calculate the percent ionization of phenol (HC6H5O) Calculate Kb For The Phenolate Ion \[ k_b = \frac{k_w}{k_a} \] We need to find the kb of format iron. To find \( k_b \) for the phenolate ion, we rearrange the equation: Your solution’s ready to go! Similarly, the equilibrium constant for the reaction of a. A solution of known concentration is prepared and its ph is measured with an. The k acid dissociation constant. Calculate Kb For The Phenolate Ion.
From sandratesneam.blogspot.com
34+ calculate the molality of phenol. SandraTesneam Calculate Kb For The Phenolate Ion Similarly, the equilibrium constant for the reaction of a. Calculating k a and k b. To find \( k_b \) for the phenolate ion, we rearrange the equation: Which base in table 14.2 ( sec. \[ k_b = \frac{k_w}{k_a} \] K b = k w k a. A solution of known concentration is prepared and its ph is measured with. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion Your solution’s ready to go! To find \( k_b \) for the phenolate ion, we rearrange the equation: The k acid dissociation constant of formic acid is more than 10 to the four negative four. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Calculating. Calculate Kb For The Phenolate Ion.
From mungfali.com
Phenol Lewis Structure Calculate Kb For The Phenolate Ion Your solution’s ready to go! The k acid dissociation constant of formic acid is more than 10 to the four negative four. K b = k w k a. Calculating k a and k b. To find \( k_b \) for the phenolate ion, we rearrange the equation: Which base in table 14.2 ( sec. A solution of known concentration. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion The k acid dissociation constant of formic acid is more than 10 to the four negative four. To find \( k_b \) for the phenolate ion, we rearrange the equation: Similarly, the equilibrium constant for the reaction of a. Your solution’s ready to go! Which base in table 14.2 ( sec. Calculating k a and k b. The numerical value. Calculate Kb For The Phenolate Ion.
From shandenlilah.blogspot.com
22+ Calculate Kb From Ph ShandenLilah Calculate Kb For The Phenolate Ion Your solution’s ready to go! Which base in table 14.2 ( sec. \[ k_b = \frac{k_w}{k_a} \] To find \( k_b \) for the phenolate ion, we rearrange the equation: We need to find the kb of format iron. The k acid dissociation constant of formic acid is more than 10 to the four negative four. We are given the. Calculate Kb For The Phenolate Ion.
From www.coursehero.com
[Solved] Phenol ionizes in water to form the phenolate ion and Calculate Kb For The Phenolate Ion The numerical value of ka and kb can be determined from an experiment. We need to find the kb of format iron. A solution of known concentration is prepared and its ph is measured with an. Which base in table 14.2 ( sec. K b = k w k a. The k acid dissociation constant of formic acid is more. Calculate Kb For The Phenolate Ion.
From www.chemistrystudent.com
Phenol (ALevel) ChemistryStudent Calculate Kb For The Phenolate Ion To find \( k_b \) for the phenolate ion, we rearrange the equation: Which base in table 14.2 ( sec. We need to find the kb of format iron. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Your solution’s ready to go! \[ k_b. Calculate Kb For The Phenolate Ion.
From www.youtube.com
The ionization constant of phenol is 1.0 X 10^10. What is the Calculate Kb For The Phenolate Ion The k acid dissociation constant of formic acid is more than 10 to the four negative four. Which base in table 14.2 ( sec. To find \( k_b \) for the phenolate ion, we rearrange the equation: Calculating k a and k b. Similarly, the equilibrium constant for the reaction of a. The numerical value of ka and kb can. Calculate Kb For The Phenolate Ion.
From www.youtube.com
Lecture 7 Ksp Common Ion Calculation YouTube Calculate Kb For The Phenolate Ion \[ k_b = \frac{k_w}{k_a} \] Similarly, the equilibrium constant for the reaction of a. K b = k w k a. We need to find the kb of format iron. A solution of known concentration is prepared and its ph is measured with an. Your solution’s ready to go! Which base in table 14.2 ( sec. To find \( k_b. Calculate Kb For The Phenolate Ion.
From www.echemi.com
How do you calculate the total phenolic contents in a plant... ECHEMI Calculate Kb For The Phenolate Ion For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). A solution of known concentration is prepared and its ph is measured with an. We need to find the kb of format iron. Calculating k a and k b. Which base in table 14.2 ( sec. The numerical value of ka. Calculate Kb For The Phenolate Ion.
From slideplayer.com
Lecture 4c Extraction. ppt download Calculate Kb For The Phenolate Ion We need to find the kb of format iron. Calculating k a and k b. For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). To find \( k_b \) for the phenolate ion, we rearrange the equation: We are given the \(pk_a\) for butyric acid and asked to calculate the. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Although the acid dissociation constant for phenol Calculate Kb For The Phenolate Ion A solution of known concentration is prepared and its ph is measured with an. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. K b = k w k a. \[ k_b = \frac{k_w}{k_a} \] We need to find the kb of format iron. Calculating. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVEDAlthough the aciddissociation constant for phenol (C6 H5 OH) is Calculate Kb For The Phenolate Ion K b = k w k a. Similarly, the equilibrium constant for the reaction of a. The numerical value of ka and kb can be determined from an experiment. A solution of known concentration is prepared and its ph is measured with an. To find \( k_b \) for the phenolate ion, we rearrange the equation: Calculating k a and. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVED Phenol (C6H6OH) has a Ka of 1.3 x 10^10. Write out the Ka Calculate Kb For The Phenolate Ion The numerical value of ka and kb can be determined from an experiment. Similarly, the equilibrium constant for the reaction of a. We need to find the kb of format iron. \[ k_b = \frac{k_w}{k_a} \] Your solution’s ready to go! A solution of known concentration is prepared and its ph is measured with an. The k acid dissociation constant. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Calculate the percent ionization of phenol (HC6H5O) Calculate Kb For The Phenolate Ion The k acid dissociation constant of formic acid is more than 10 to the four negative four. K b = k w k a. \[ k_b = \frac{k_w}{k_a} \] Your solution’s ready to go! To find \( k_b \) for the phenolate ion, we rearrange the equation: Which base in table 14.2 ( sec. We are given the \(pk_a\) for. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion Your solution’s ready to go! Calculating k a and k b. Which base in table 14.2 ( sec. Similarly, the equilibrium constant for the reaction of a. K b = k w k a. A solution of known concentration is prepared and its ph is measured with an. We are given the \(pk_a\) for butyric acid and asked to calculate. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVED Phenol, C6H5OH, is an aromatic alcohol with weak basic Calculate Kb For The Phenolate Ion The numerical value of ka and kb can be determined from an experiment. K b = k w k a. The k acid dissociation constant of formic acid is more than 10 to the four negative four. Similarly, the equilibrium constant for the reaction of a. For an aqueous solution of a weak acid, the dissociation constant is called the. Calculate Kb For The Phenolate Ion.
From www.toppr.com
The ionization constant of phenol is 1.0 × 10^10 . What is the Calculate Kb For The Phenolate Ion The k acid dissociation constant of formic acid is more than 10 to the four negative four. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Calculating k a and k b. For an aqueous solution of a weak acid, the dissociation constant is called. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVED 16. a) Sodium phenolate (C6H5ONa) is a salt that contains the Calculate Kb For The Phenolate Ion We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. Similarly, the equilibrium constant for the reaction of a. The numerical value of ka and kb can be determined from an experiment. Your solution’s ready to go! \[ k_b = \frac{k_w}{k_a} \] We need to find. Calculate Kb For The Phenolate Ion.
From www.slideserve.com
PPT Phenols PowerPoint Presentation ID735552 Calculate Kb For The Phenolate Ion To find \( k_b \) for the phenolate ion, we rearrange the equation: A solution of known concentration is prepared and its ph is measured with an. Similarly, the equilibrium constant for the reaction of a. The k acid dissociation constant of formic acid is more than 10 to the four negative four. The numerical value of ka and kb. Calculate Kb For The Phenolate Ion.
From www.numerade.com
SOLVED Calculate the percent ionized in 7.67x10^6 M phenol a very Calculate Kb For The Phenolate Ion To find \( k_b \) for the phenolate ion, we rearrange the equation: \[ k_b = \frac{k_w}{k_a} \] For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). We need to find the kb of format iron. Similarly, the equilibrium constant for the reaction of a. The numerical value of ka. Calculate Kb For The Phenolate Ion.
From www.chegg.com
Solved Recognize physical and chemical properties of phenols Calculate Kb For The Phenolate Ion The numerical value of ka and kb can be determined from an experiment. Which base in table 14.2 ( sec. Your solution’s ready to go! We need to find the kb of format iron. We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. K b. Calculate Kb For The Phenolate Ion.
From www.coursehero.com
[Solved] Phenol ionizes in water to form the phenolate ion and Calculate Kb For The Phenolate Ion \[ k_b = \frac{k_w}{k_a} \] To find \( k_b \) for the phenolate ion, we rearrange the equation: We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. The k acid dissociation constant of formic acid is more than 10 to the four negative four. We. Calculate Kb For The Phenolate Ion.
From ar.inspiredpencil.com
Phenol Lewis Structure Calculate Kb For The Phenolate Ion We are given the \(pk_a\) for butyric acid and asked to calculate the \(k_b\) and the \(pk_b\) for its conjugate base, the butyrate ion. We need to find the kb of format iron. A solution of known concentration is prepared and its ph is measured with an. Calculating k a and k b. The numerical value of ka and kb. Calculate Kb For The Phenolate Ion.
From www.researchgate.net
How to calculate of total phenolic and total flavonoid content Calculate Kb For The Phenolate Ion Calculating k a and k b. The numerical value of ka and kb can be determined from an experiment. For an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\ (k_a\)). A solution of known concentration is prepared and its ph is measured with an. The k acid dissociation constant of formic acid. Calculate Kb For The Phenolate Ion.