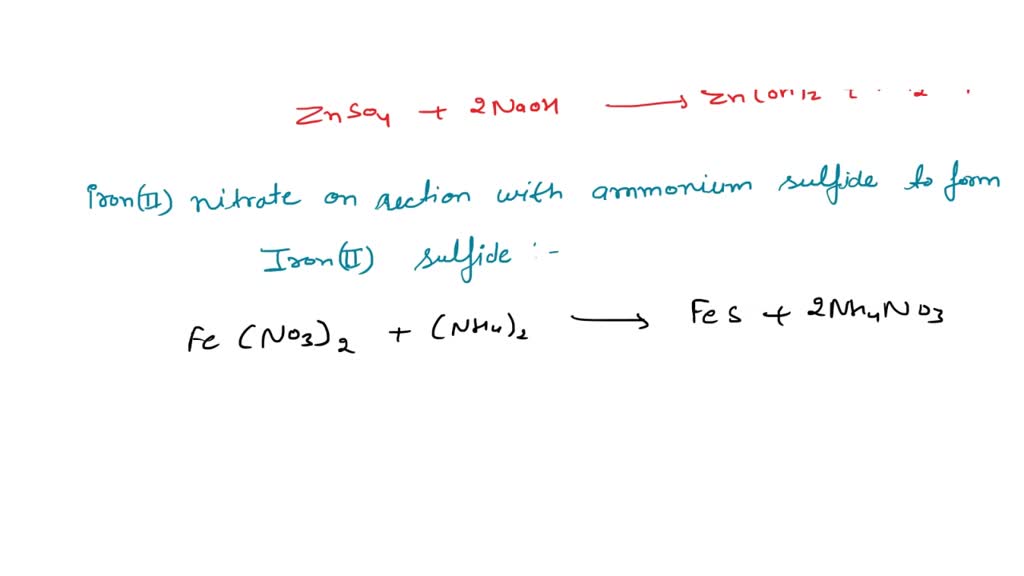
Zinc Sulfate And Sodium Sulfide Precipitate . When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Because both components of each. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. This guide will show how to use the solubility rules. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Na2s + znso4 = na2so4 + zns is a double displacement.
from www.numerade.com
Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Because both components of each. This guide will show how to use the solubility rules. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous.
SOLVED Does precipitate form when A and B are mixed? empirical formula
Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Na2s + znso4 = na2so4 + zns is a double displacement. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Because both components of each.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Sulfate And Sodium Sulfide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Sodium chloride, sodium hydroxide, or sodium sulfate?. Na2s + znso4 = na2so4 + zns is a. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.researchgate.net
Zeta potential of zinc sulfide precipitates (circles) and mineral Zinc Sulfate And Sodium Sulfide Precipitate Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Na2s + znso4 = na2so4 + zns is a double displacement. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: When two aqueous solutions of ionic compounds are mixed together, the resulting reaction. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
SOLVED Texts Complete the table below by deciding whether a Zinc Sulfate And Sodium Sulfide Precipitate Na2s + znso4 = na2so4 + zns is a double displacement. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. Enter an ionic equation (solubility states are optional) to calculate. Zinc Sulfate And Sodium Sulfide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Sulfate And Sodium Sulfide Precipitate Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. This guide will show how to use the solubility rules. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Enter. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Zinc Sulfate And Sodium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Na2s + znso4 = na2so4 + zns is a double displacement. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Enter an. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. This guide will show how to use the. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.sciencephoto.com
Zinc sulphide precipitate, 3 of 3 Stock Image C036/3906 Science Zinc Sulfate And Sodium Sulfide Precipitate Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Which solution could be used. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.youtube.com
Reaction of Sodium sulphide (Na2S) and zinc sulphate (ZnSO4) YouTube Zinc Sulfate And Sodium Sulfide Precipitate This guide will show how to use the solubility rules. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. When two aqueous solutions of. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
SOLVED Zinc ions made/should have made a precipitate with which of Zinc Sulfate And Sodium Sulfide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Na2s + znso4 = na2so4 + zns is a double displacement. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. The precipitate is then removed by filtration and the. Zinc Sulfate And Sodium Sulfide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Sulfate And Sodium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Enter. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.toppr.com
Given below are word equations. Convert them into chemical equations Zinc Sulfate And Sodium Sulfide Precipitate Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Which solution could be used to precipitate the barium ion,. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Zinc Sulfate And Sodium Sulfide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Because both components of each. This guide will show how to use the solubility rules. Sodium chloride, sodium hydroxide, or sodium sulfate?.. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.researchgate.net
(A) Pictures of the microwave assisted zinc sulfide nanoparticle Zinc Sulfate And Sodium Sulfide Precipitate This guide will show how to use the solubility rules. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Na2s + znso4 = na2so4 + zns is a double displacement. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of. Zinc Sulfate And Sodium Sulfide Precipitate.
From questions.kunduz.com
Complete the table below by deciding whet... Physical Chemistry Zinc Sulfate And Sodium Sulfide Precipitate Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Because both components of. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.sciencephoto.com
Zinc sulphide precipitate, 1 of 3 Stock Image C036/3904 Science Zinc Sulfate And Sodium Sulfide Precipitate Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Which of the following reaction correctly represents the. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.youtube.com
Double displacement of ZnSO4 + BaCl2 Zinc sulphate + Barium chloride Zinc Sulfate And Sodium Sulfide Precipitate Sodium chloride, sodium hydroxide, or sodium sulfate?. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Enter. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.mdpi.com
ChemEngineering Free FullText Removal of Metals by Sulphide Zinc Sulfate And Sodium Sulfide Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Na2s + znso4 = na2so4 + zns is. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
A solution of Iron (II) nitrate is poured into a sodium sulfide Zinc Sulfate And Sodium Sulfide Precipitate This guide will show how to use the solubility rules. Na2s + znso4 = na2so4 + zns is a double displacement. Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Precipitation reactions are a subclass of exchange. Zinc Sulfate And Sodium Sulfide Precipitate.
From sciengineeredmaterials.com
Zinc Sulfide Data Sheet SCI Engineered Materials Zinc Sulfate And Sodium Sulfide Precipitate Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. This guide will show how to use the solubility rules. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Which solution could be used to precipitate the. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.shutterstock.com
79 Residual Volume Images, Stock Photos & Vectors Shutterstock Zinc Sulfate And Sodium Sulfide Precipitate Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. This guide will show how to use the solubility rules. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.youtube.com
Does sodium sulfide (Na2S) and zinc nitrate (Zn(NO3)2 precipitate Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. This guide will show. Zinc Sulfate And Sodium Sulfide Precipitate.
From dmishin.github.io
Sodium Zinc Sulfate Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.slideserve.com
PPT Double Replacement 1. Hydrogen sulfide is bubbled through a Zinc Sulfate And Sodium Sulfide Precipitate The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Which solution could be used to precipitate the barium ion, ba 2+, in a water. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.researchgate.net
a Color change and formation of white precipitate, b mechanism of Zinc Sulfate And Sodium Sulfide Precipitate Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m sodium sulfide produced 0.279 g of a precipitate. Sodium chloride, sodium hydroxide, or sodium sulfate?. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Na2s + znso4 = na2so4 + zns is a. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.coursehero.com
[Solved] 5. Precipitation Reaction. Sodium sulfide is commonly used to Zinc Sulfate And Sodium Sulfide Precipitate This guide will show how to use the solubility rules. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2 in a recarbonation process. Which of the following reaction. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.researchgate.net
Relationship of pH and zinc sulphide precipitation process. Download Zinc Sulfate And Sodium Sulfide Precipitate Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. This guide will show how to use the solubility rules. Because both components of each. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which of. Zinc Sulfate And Sodium Sulfide Precipitate.
From dmishin.github.io
Sodium Zinc Sulfate Zinc Sulfate And Sodium Sulfide Precipitate Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Na2s + znso4 = na2so4 + zns is a double displacement. Because both components of each. When two aqueous solutions of ionic compounds are. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.toppr.com
Given below are word equations. Convert them into chemical equations Zinc Sulfate And Sodium Sulfide Precipitate Because both components of each. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Sodium chloride, sodium hydroxide, or sodium sulfate?. When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when. Zinc Sulfate And Sodium Sulfide Precipitate.
From frgmnt.org
NaPS Etchant Chemistry « frgmnt org Zinc Sulfate And Sodium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. This guide will show how to use the solubility rules. Adding 10.0 ml of a dilute solution of zinc nitrate to 246 ml of 2.00 m. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.chegg.com
Solved does zinc bromide and sodium sulfide precipitate and Zinc Sulfate And Sodium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Zinc Sulfate And Sodium Sulfide Precipitate Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Because both components of each. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. The precipitate is then removed by filtration and the water is brought back to a neutral ph by the addition of co 2. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.numerade.com
SOLVED Does precipitate form when A and B are mixed? empirical formula Zinc Sulfate And Sodium Sulfide Precipitate When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Na2s + znso4 = na2so4 +. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Sulfate And Sodium Sulfide Precipitate Which of the following reaction correctly represents the precipitation reaction between sodium sulfide and zinc sulfate in aqueous. Sodium chloride, sodium hydroxide, or sodium sulfate?. Which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Na2s + znso4 = na2so4 + zns is a double displacement. Adding 10.0 ml of a dilute solution of. Zinc Sulfate And Sodium Sulfide Precipitate.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Sulfate And Sodium Sulfide Precipitate Na2s + znso4 = na2so4 + zns is a double displacement. Precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Sodium sulfide + zinc sulfate = sodium sulfate + zinc sulfide. Sodium chloride, sodium hydroxide, or sodium sulfate?. Enter an ionic equation (solubility states are optional) to calculate its. Zinc Sulfate And Sodium Sulfide Precipitate.